|
Boron Compounds
Boron compounds are compounds containing the element boron. In the most familiar compounds, boron has the formal oxidation state +3. These include oxides, sulfides, nitrides, and halides. Halides The trihalides adopt a planar trigonal structure. These compounds are Lewis acids in that they readily form adducts with electron-pair donors, which are called Lewis bases. For example, fluoride (F−) and boron trifluoride (BF3) combined to give the tetrafluoroborate anion, BF4−. Boron trifluoride is used in the petrochemical industry as a catalyst. The halides react with water to form boric acid. Boron is found in nature on Earth almost entirely as various oxides of B(III), often associated with other elements. More than one hundred borate minerals contain boron in oxidation state +3. These minerals resemble silicates in some respect, although boron is often found not only in a tetrahedral coordination with oxygen, but also in a trigonal planar configuration. Unlike silicates, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis Product
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and ''lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves , a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these clusters are polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes. In terms of scope, carboranes can have as few as 5 and as many as 14 atoms in the cage framework. The majority have two cage carbon atoms. The corresponding C-alkyl and B-alkyl analogues are also known in a few cases. Structure and bonding Carboranes and boranes adopt 3-dimensional cage (cluster) geometries in sharp contrast to typical organic compounds. Cages are compatible with sigma—delocalized bonding, whereas hydrocarbons are typically chains or rings. Like for other electron-delocalized polyhedral clusters, the electronic structure of these cluster compounds can be described by the Wade–Mingos rules. Like the related boron hydrides, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond. History Boron carbide was discovered in the 19th century as a by-product of reactions involving metal borides, but its chemical formula was unknown. It was not until the 1930s that the chemical composition was estimated as B4C. Controversy remained as to whether or not the material had this exact 4:1 stoichiometry, as, in practice the material is always slightly carbon-deficient with regard to this formula, and X-ray crystallography shows that its structure is highly complex, with a mixture of C-B-C chains and B12 icosahedra. These features argued against a very simple exact B4C empirical formula. Because of the B12 structural unit, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylborane
Triphenylborane, often abbreviated to BPh3 where Ph is the phenyl group C6H5-, is a chemical compound with the formula B(C6H5)3. It is a white crystalline solid and is both air and moisture sensitive, slowly forming benzene and triphenylboroxine. It is soluble in aromatic solvents. Structure and properties The core of the compound, BC3, has a trigonal planar structure. The phenyl groups are rotated at about a 30° angle from the core plane. Even though triphenylborane and tris(pentafluorophenyl)borane are structurally similar, their Lewis acidity is not. BPh3 is a weak Lewis acid while B(C6F5)3 is a strong Lewis acid due to the electronegativity of the fluorine atoms. Other boron Lewis acids include BF3 and BCl3. Synthesis Triphenylborane was first synthesized in 1922. It is typically made with boron trifluoride diethyl etherate and the Grignard reagent, phenylmagnesium bromide. : BF3•O(C2H5)2 + 3 C6H5MgBr → B(C6H5)3 + 3 MgBrF + (C2H5)2O Triphenylborane can also ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraphenylborate
Tetraphenylborate (IUPAC name: Tetraphenylboranuide) is an organoboron anion consisting of a central boron atom with four phenyl groups. Salts of tetraphenylborate uncouple oxidative phosphorylation. See also *Sodium tetraphenylborate *Potassium tetraphenylborate *Triphenylborane * BARF and other fluorinated derivatives are used as non-coordinating anions Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly fou .... References Anions {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
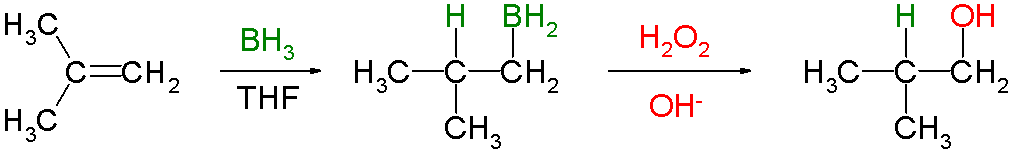
Hydroboration–oxidation Reaction
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979. The general form of the reaction is as follows: Tetrahydrofuran (THF) is the archetypal solvent used for hydroboration. Mechanism and scope Hydroboration step In the first step, borane (BH3) adds to the double bond, transferring one of the hydrogen atoms to the carbon adjacent to the one that becom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordinate Covalent Bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal ions to ligands involves this kind of interaction. This type of interaction is central to Lewis acid–base theory. Coordinate bonds are commonly found in coordination compounds. Examples Coordinate covalent bonding is ubiquitous. In all metal aquo-complexes (H2O)''n'''m''+, the bonding between water and the metal cation is described as a coordinate covalent bond. Metal-ligand interactions in most organometallic compounds and most coordination compounds are described similarly. The term ''dipolar bond'' is used in organic chemistry for compounds such as amine oxides for which the electronic structure can be described in terms of the basic amine donating two electrons to an oxygen atom. : → O The arrow → indicates that both ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borazon
Borazon is a brand name of a cubic form of boron nitride (cBN). Its color ranges from black to brown and gold, depending on the chemical bond. It is one of the hardest known materials, along with various forms of diamond and kinds of boron nitride. Borazon is a crystal created by heating equal quantities of boron and nitrogen at temperatures greater than 1800 °C (3300 °F) at 7 GPa (1 million lbf/in2). Borazon was first produced in 1957 by Robert H. Wentorf, Jr., a physical chemist working for the General Electric Company. In 1969, General Electric adopted the name Borazon as its trademark for the material. The trademark is now owned by Diamond Innovations, doing business aHyperion Materials & Technologies, Inc. and Borazon is manufactured only by Hyperion Materials & Technologies. Uses and production Borazon has a number of uses , such as: cutting tools, dies, punches, shears, knives, saw blades, bearing rings, needles, rollers, spacers, balls, pump, compres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allotropes Of Carbon
Carbon is capable of forming many allotropy, allotropes (structurally different forms of the same element) due to its Valence (chemistry), valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include carbon nano tube, nanotubes, Carbon nanobud, nanobuds and Graphene nanoribbon, nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Diamond Diamond is a well-known allotrope of carbon. The hardness, extremely high refractive index, and high Dispersion (optics), dispersion of light make diamond useful for industrial applications and for jewelry. Diamond is the hardest known natural min ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varyin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |