|
Carborane
Carboranes (or carbaboranes) are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these clusters are Polyhedron, polyhedra or fragments of polyhedra. Carboranes are one class of heteroboranes. In terms of scope, carboranes can have as few as 5 and as many as 14 atoms in the cage framework. The majority have two cage carbon atoms. The corresponding carbon, C-alkyl and boron, B-alkyl analogues are also known in a few cases. Structure and bonding Carboranes and boranes adopt 3-dimensional cage (Cluster chemistry, cluster) geometries in sharp contrast to typical organic compounds. Cages are compatible with sigma—delocalized bonding, whereas hydrocarbons are typically chains or rings. Like for other electron-delocalized polyhedral clusters, the electronic structure of these cluster compounds can be described by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heteroborane
Heteroboranes are classes of boranes in which at least one boron atom is replaced by another Chemical element, elements. Like many of the related boranes, these clusters are polyhedra and are similarly classified as Boranes#Chemical formula and naming conventions, ''closo-'', ''nido-'', ''arachno-'', and ''hypho-'', according to the so-called Polyhedral skeletal electron pair theory, electron count. ''Closo-'' represents a complete polyhedron, while ''nido-'', ''arachno-'' and ''hypho-'' stand for polyhedrons that are missing one, two and three vertices. Besides carbon (carboranes or carbaboranes), other elements can also be included in the heteroborane molecules as well, such as silicon, Si (silaboranes), nitrogen, N (azaboranes, including borazine), phosphorus, P (phosphaboranes), arsenic, As (arsaboranes), antimony, Sb (stibaboranes), oxygen, O (oxaboranes), sulfur, S (thiaboranes), selenium, Se (selenaboranes) and tellurium, Te (telluraboranes), either alone or in combination. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
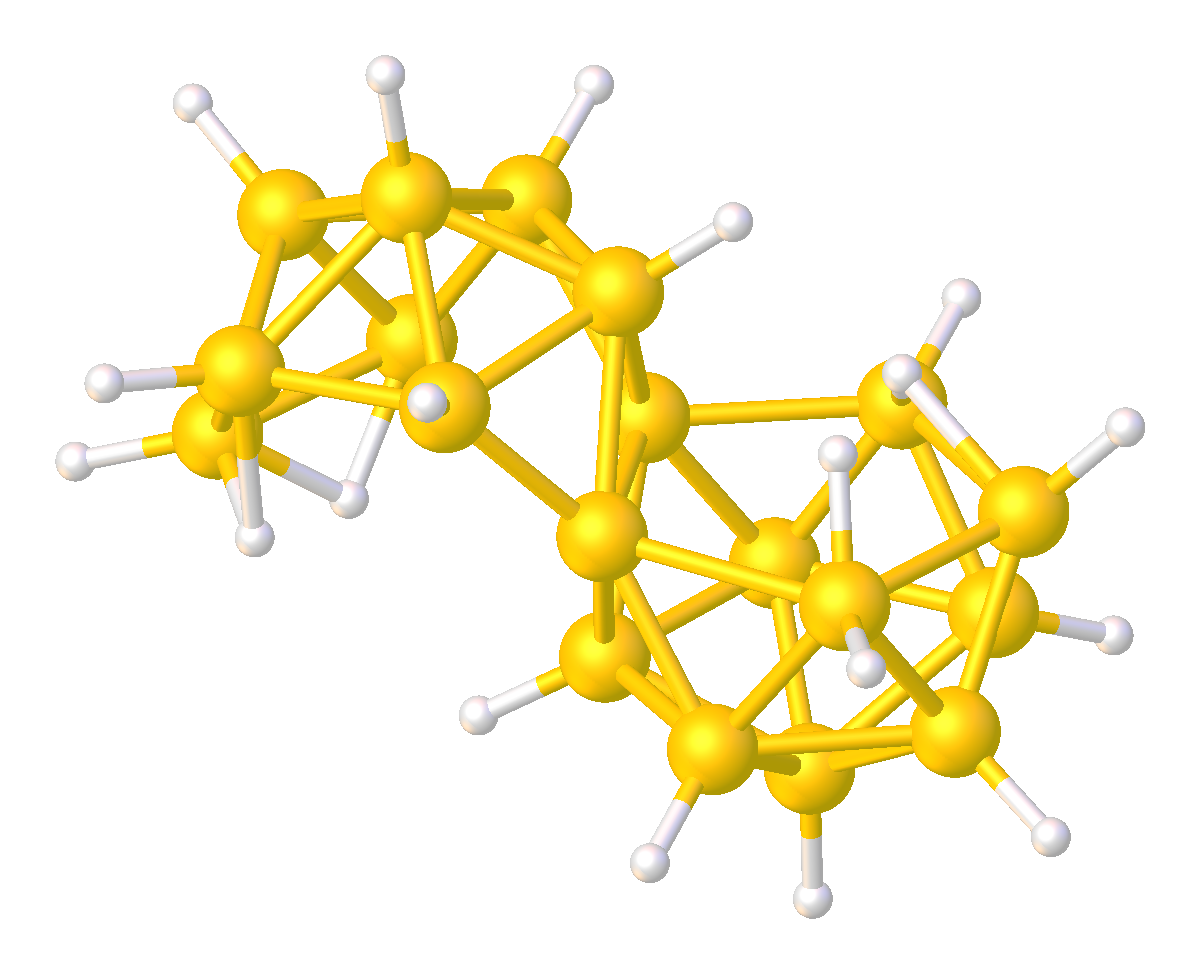
Dodecaborate
The dodecaborate(12) anion, 12H12sup>2−, is a borane with an icosahedral arrangement of 12 boron atoms, with each boron atom being attached to a hydrogen atom. Its symmetry is classified by the molecular point group Ih. Synthesis and reactions The existence of the dodecaborate(12) anion, 12H12sup>2−, was predicted by H. C. Longuet-Higgins and M. de V. Roberts in 1955. Hawthorne and Pitochelli first made it 5 years later, by the reaction of 2-iododecaborane with triethylamine in benzene solution at 80 °C. It is more conveniently prepared in two steps from sodium borohydride. First the borohydride is converted into a triborate anion using the etherate of boron trifluoride: : 5 NaBH4 + BF3 → 2 NaB3H8 + 3 NaF + 2 H2 Pyrolysis of the triborate gives the twelve-boron cluster as the sodium salt. A variety of other synthetic methods have been published. Salts of the dodecaborate ion are stable in air and do not react with hot aqueous sodium hydroxide or hydrochlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, carbon-12, C and carbon-13, C being stable, while carbon-14, C is a radionuclide, decaying with a half-life of 5,700 years. Carbon is one of the timeline of chemical element discoveries#Pre-modern and early modern discoveries, few elements known since antiquity. Carbon is the 15th abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the abundance of the chemical elements, fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon's abundance, its unique diversity of organic compounds, and its unusual abi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Closo Cluster
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of cluster compound, clusters such as Boranes, borane and carborane clusters. The electron counting rules were originally formulated by Kenneth Wade, and were further developed by others including Michael Mingos; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital treatment of the bonding. These notes contained original material that served as the basis of the sections on the 4''n'', 5''n'', and 6''n'' rules. These rules have been extended and unified in the form of the Jemmis mno rules, Jemmis ''mno'' rules. Predicting structures of cluster compounds Different rules (4''n'', 5''n'', or 6''n'') are invoked depending on the number of electrons per vertex. The 4''n'' rules are reasonably accurate in predicting the structures of clusters having about 4 electrons per vertex, as is the case ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Hydrides
Boron hydride clusters are compounds with the formula or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed. History The development of the borane hydride clusters resulted from pioneering work by Alfred Stock, invented the glass vacuum line for their study. The structures of the boron hydride clusters were determined beginning in 1948 with the characterization of decaborane. William Lipscomb was awarded the Nobel Prize in Chemistry in 1976 for this and many subsequent crystallographic investigations. These investigations revealed the prevalence of deltahedral structures, i.e., networks of triangular arrays of BH centers. The bonding of the clusters ushered i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decaborane
Decaborane, also called decaborane(14), is the inorganic compound with the chemical formula B10 H14. It is classified as a borane and more specifically a boron hydride cluster. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides. It is toxic and volatile, giving off a foul odor, like that of burnt rubber or chocolate. Handling, properties and structure The physical characteristics of decaborane(14) resemble those of naphthalene and anthracene, all three of which are volatile colorless solids. Sublimation is the common method of purification. Decaborane is highly flammable, and burns with a bright green flame like other boron hydrides. It is not sensitive to moist air, although it hydrolyzes in boiling water, releasing hydrogen and giving a solution of boric acid. It is soluble in cold water as well as a variety of non-polar and moderately polar solvents. In decaborane, the B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyhedral Skeletal Electron Pair Theory
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by Kenneth Wade, and were further developed by others including Michael Mingos; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital treatment of the bonding. These notes contained original material that served as the basis of the sections on the 4''n'', 5''n'', and 6''n'' rules. These rules have been extended and unified in the form of the Jemmis ''mno'' rules. Predicting structures of cluster compounds Different rules (4''n'', 5''n'', or 6''n'') are invoked depending on the number of electrons per vertex. The 4''n'' rules are reasonably accurate in predicting the structures of clusters having about 4 electrons per vertex, as is the case for many boranes and carboranes. For su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weakly Coordinating Anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations C5H5)2ZrRsup>+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (). As a precursor to many other materials and chemical compounds, in 2006 the global production of formaldehyde was estimated at 12 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Formaldehyde also occurs naturally. It is derived from the degradation of serine, dimethylglycine, and lipids. Demethylases act by converting N-methyl groups to formaldehyde. Formaldehyde is classified as a group 1 carcinogen and can cause respiratory and skin irritation upon exposure. Forms Formaldehyde is more complicated than many simple carbon compounds in that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borane Dimethylsulfide
Borane dimethylsulfide (BMS) is a chemical compound with the chemical formula . It is an adduct between borane molecule () and dimethyl sulfide molecule (). It is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart (10 M) and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, Borane–tetrahydrofuran, requires sodium borohydride to inhibit reduction of THF to tributyl borate (). BMS is soluble in most aprotic solvents. Preparation and structure Although usually purchased, BMS can be prepared by absorbing diborane into dimethyl sulfide: : It can be purified by bulb to bulb vacuum transfer. Although a structure of BMS has not been determined crystallographically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |