|
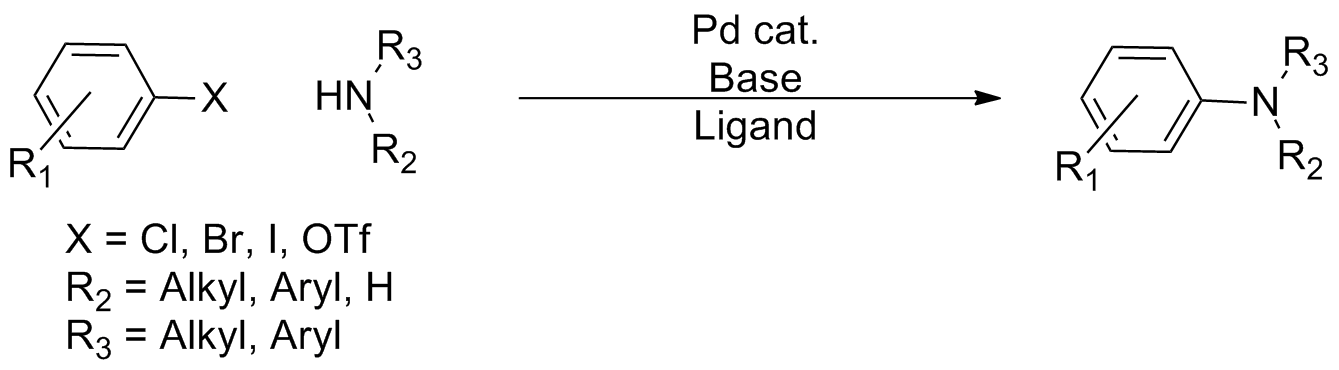
Buchwald–Hartwig Amination
In organic chemistry, the Buchwald–Hartwig amination is a chemical reaction for the synthesis of carbon–nitrogen bonds via the cross-coupling reaction, palladium-catalyzed coupling reactions of amines with aryl halides. Although Pd-catalyzed C-N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods (nucleophilic substitution, reductive amination, etc.) for the synthesis of aromatic bonds, with most methods suffering from limited substrate scope and functional group tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods (the Goldberg reaction, nucleophilic aromatic substitution, etc.) while significantly expanding the repertoire of possible bond formatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stephen L
Stephen or Steven is a common English first name. It is particularly significant to Christians, as it belonged to Saint Stephen ( grc-gre, Στέφανος ), an early disciple and deacon who, according to the Book of Acts, was stoned to death; he is widely regarded as the first martyr (or "protomartyr") of the Christian Church. In English, Stephen is most commonly pronounced as ' (). The name, in both the forms Stephen and Steven, is often shortened to Steve or Stevie. The spelling as Stephen can also be pronounced which is from the Greek original version, Stephanos. In English, the female version of the name is Stephanie. Many surnames are derived from the first name, including Stephens, Stevens, Stephenson, and Stevenson, all of which mean "Stephen's (son)". In modern times the name has sometimes been given with intentionally non-standard spelling, such as Stevan or Stevon. A common variant of the name used in English is Stephan ; related names that have found some curr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Withdrawing Group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications: *with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the appended species. Tetracyanoethylene is an oxidant because the alkene is appended to four cyano substituents, which are electron-withdrawing. *with regards to acid-base reactions, acids with electron-withdrawing groups species have low acid dissociation constants. For EWG's attached to benzene, this effect is described by the Hammett equation, which allows EWGs to be discussed quantitatively. *with regards to nucleophilic substitution reactions, electron-withdrawing groups are susceptible to attack by weak nucleophiles. For example, compared to chlorobenzene, chlorodinitrobenzene is susceptible to reactions that displace chloride.{{cite journal , author=J. F. Bunnett, R. M. Conner, doi=10.15227/orgsyn.040.0034, title=2,4-Dinitroiodobe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylamine
Diethylamine is an organic compound with the formula (CH3CH2)2NH. It is a secondary amine. It is a flammable, weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to impurities. It has a strong ammonia-like odor. Production and uses Diethylamine is made by the alumina-catalyzed reaction of ethanol and ammonia. It is obtained together with ethylamine and triethylamine. Annual production of three ethylamines was estimated in 2000 to be 80,000,000 kg. It is used in the production of corrosion inhibitor ''N'',''N''- diethylaminoethanol, by reaction with ethylene oxide. It is also a precursor to a wide variety of other commercial products. Diethylamine is also sometimes used in the illicit production of LSD. Supramolecular structure Diethylamine is the smallest and simplest molecule that features a supramolecular helix as its lowest energy aggregate. Other similarly sized hydrogen-bonding In c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all of the argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete octe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids (amino acids that can be synthesized de novo by the organism). Transamination in biochemistry is accomplished by enzymes called transaminases or aminotransferases. α-ketoglutarate acts as the predominant amino-group acceptor and produces glutamate as the new amino acid. :Aminoacid + α-ketoglutarate ↔ α-keto acid + glutamate Glutamate's amino group, in turn, is transferred to oxaloacetate in a second transamination reaction yielding aspartate. :Glutamate + oxaloacetate ↔ α-ketoglutarate + aspartate Mechanism of action Transamination catalyzed by aminotransferase occurs in two stages. In the first step, the α amino group of an amino acid is transferred to the enzyme, producing the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
D Orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital, p orbit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Letters
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 2.415. Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Publications established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrakis Triphenylphosphine Palladium
Tetrakis(triphenylphosphine)palladium(0) (sometimes called quatrotriphenylphosphine palladium) is the chemical compound d(P(C6H5)3)4 often abbreviated Pd( PPh3)4, or rarely PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air. Structure and properties The four phosphorus atoms are at the corners of a tetrahedron surrounding the palladium(0) center. This structure is typical for four-coordinate 18 e− complexes. The corresponding complexes Ni(PPh3)4 and Pt(PPh3)4 are also well known. Such complexes reversibly dissociate PPh3 ligands in solution, so reactions attributed to Pd(PPh3)4 often in fact arise from Pd(PPh3)3 or even Pd(PPh3)2. Preparation Tetrakis(triphenylphosphine)palladium(0) was first prepared by Lamberto Malatesta et al. in the 1950s by reduction of sodium chloropalladate with hydrazine in the presence of the phosphine. It is commercially available, but can be prepared in two steps from Pd(II) precursors: :PdCl2 + 2 PPh3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |