|
Bis(cyclooctadiene)nickel(0)
Bis(cyclooctadiene)nickel(0) is the organonickel compound with the formula Ni(C8H12)2, also written Ni(cod)2. It is a diamagnetic coordination complex featuring tetrahedral nickel(0) bound to the alkene groups in two 1,5-cyclooctadiene ligands. This highly air-sensitive yellow solid is a common source of Ni(0) in chemical synthesis. Preparation and properties The complex is prepared by reduction of anhydrous nickel(II) acetylacetonate in the presence of the diolefin: :Ni(acac)2 + 2 cod + 2 AlEt3 → Ni(cod)2 + 2 acacAlEt2 + C2H6 + C2H4 Ni(cod)2 is moderately soluble in several organic solvents. One or both 1,5-cyclooctadiene ligands are readily displaced by phosphines, phosphites, bipyridine, and isocyanides. If exposed to air, the solid oxidizes to nickel(II) oxide. As a result, this compound is generally handled in a glovebox A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organonickel Compounds
Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process. Classes of compounds : Alkyl and aryl complexes A popular reagent is Tetramethylethylenediamine(dimethyl)nickel(II), Ni(CH3)2(tetramethylethylenediamine). Many alkyl and aryl complexes are known with the formula NiR(X)L2. Examples include [(dppf)Ni(cinnamyl)Cl)], ''trans''-(PCy2Ph)2Ni(''o''-tolyl)Cl, (dppf)Ni(''o''-tolyl)Cl, (TMEDA)Ni(''o''-tolyl)Cl, and (TMEDA)NiMe2. Nickel co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organonickel Compound
Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process. Classes of compounds : Alkyl and aryl complexes A popular reagent is Ni(CH3)2(tetramethylethylenediamine). Many alkyl and aryl complexes are known with the formula NiR(X)L2. Examples include dppf)Ni(cinnamyl)Cl) ''trans''-(PCy2Ph)2Ni(''o''-tolyl)Cl, (dppf)Ni(''o''-tolyl)Cl, (TMEDA)Ni(''o''-tolyl)Cl, and (TMEDA)NiMe2. Nickel compounds of the type NiR2 also exist with just 12 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,5-cyclooctadiene
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula , specifically . There are three configurational isomers with this structure, that differ by the arrangement of the four C–C single bonds adjacent to the double bonds. Each pair of single bonds can be on the same side () or on opposite sides () of the double bond's plane; the three possibilities are denoted , , and ; or (), (), and (). (Because of overall symmetry, is the same configuration as .) Generally abbreviated COD, the isomer of this diene is a useful precursor to other organic compounds and serves as a ligand in organometallic chemistry. It is a colorless liquid with a strong odor. 1,5-Cyclooctadiene can be prepared by dimerization of butadiene in the presence of a nickel catalyst, a coproduct being vinylcyclohexene. Approximately 10,000 tons were produced in 2005. Organic reactions COD reacts with borane to give 9-borabicyclo .3.1onane, commonly known as 9-BBN, a reagent in organic chemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylaluminium
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( C2H5)6 (abbreviated as Al2Et6 or TEA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to trimethylaluminium. Structure and bonding The structure and bonding in Al2R6 and diborane are analogous (R = alkyl). Referring to Al2Me6, the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral. The carbon atoms of the bridging ethyl groups are each surrounded by five neighbors: carbon, two hydrogen atoms and two aluminium atoms. The ethyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric AlEt3. Synthesis and reactions Triethylaluminium can be formed via several routes. The discovery of an efficient route was a significant technological achievement. The multistep process uses aluminium m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glovebox
A glovebox (or glove box) is a sealed container that is designed to allow one to manipulate objects where a separate atmosphere is desired. Built into the sides of the glovebox are gloves arranged in such a way that the user can place their hands into the gloves and perform tasks inside the box without breaking containment. Part or all of the box is usually transparent to allow the user to see what is being manipulated. Two types of gloveboxes exist. The first allows a person to work with hazardous substances, such as radioactive materials or infectious disease agents, and the second allows manipulation of substances that must be contained within a very high purity inert atmosphere, such as argon or nitrogen. It is also possible to use a glovebox for manipulation of items in a vacuum chamber. Inert atmosphere work The gas in a glovebox is pumped through a series of treatment devices which remove solvents, water and oxygen from the gas. Copper metal (or some other finely divi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Oxide
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful representations. They are sus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
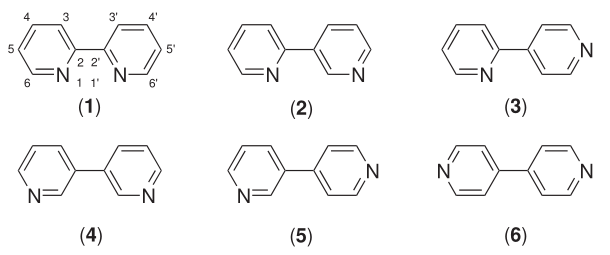
Bipyridine
Bipyridines also known as bipyridyls, dipyridyls, and dipyridines, are a family of chemical compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. Bipyridines are of significance in pesticides. Six isomers of bipyridine exist, but two are prominent: 2,2′-bipyridine is a popular ligand. 4,4'-Bipyridine is a precursor to the commercial herbicide paraquat. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. 2,2′-Bipyridine 2,2′-Bipyridine (2,2′-bipy) is a chelating ligand that forms complexes with most transition metal ions that are of broad academic interest. Many of these complexes have distinctive optical properties, and some are of interest for analysis. Its complexes are used in studies of electron and energy transfer, supramolecular and materials chemistry, and catalysis. 2,2′-Bipyridine is used in the manufacture of diqua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Syntheses
''Inorganic Syntheses'' is a book series which aims to publish "detailed and foolproof" procedures for the synthesis of inorganic compounds. Although this series of books are edited, they usually are referenced like a journal, without mentioning the names of the checkers (referees) or the editor. A similar format is usually followed for the series ''''. Volumes See also *Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. I ...
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Acetylacetonate
Nickel(II) bis(acetylacetonate) is a coordination complex with the formula i(acac)2sub>3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2. Structure and properties Anhydrous nickel(II) acetylacetonate exists as molecules of Ni3(acac)6. The three nickel atoms are approximately collinear and each pair of them is bridged by two μ2 oxygen atoms. Each nickel atom has tetragonally distorted octahedral geometry, caused by the difference in the length of the Ni-O bonds between the bridging and non-bridging oxygens. Ni3(acac)6 molecules are almost centrosymmetric, despite the non-centrosymmetric point group of the ''cis''-Ni(acac)2 "monomers," which is uncommon. The trimeric structure allows all nickel centers to achieve an octahedral coordination. The trimer is only formed if intramolecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing ''n''-butane to crude maleic anhydride, followed by catalytic hydrogenation. A third major industrial route entails hydroformylation of allyl alcohol followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2.png)




2.png)
2(H2O)2.png)
