1,5-Cyclooctadiene on:
[Wikipedia]
[Google]
[Amazon]
Cycloocta-1,5-diene is a
 COD adds (or similar reagents) to give 2,6-dichloro-9-thiabicyclo .3.1onane:
:
COD adds (or similar reagents) to give 2,6-dichloro-9-thiabicyclo .3.1onane:
: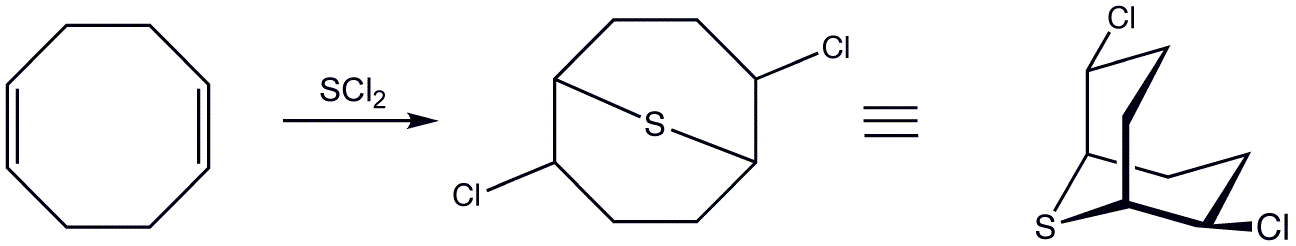 The resulting dichloride can be further modified as the di azide or di
The resulting dichloride can be further modified as the di azide or di
File:Ni(cod)2.png, Bis(cyclooctadiene)nickel(0).
File: Crabtree.svg,
1,5-COD binds to low-valent metals via both alkene groups. Metal-COD complexes are attractive because they are sufficiently stable to be isolated, often being more robust than related ethylene complexes. The stability of COD complexes is attributable to the
 The highly strained ''trans'',''trans'' isomer of 1,5-cyclooctadiene is a known compound. (''E'',''E'')-COD was first synthesized by
The highly strained ''trans'',''trans'' isomer of 1,5-cyclooctadiene is a known compound. (''E'',''E'')-COD was first synthesized by
cyclic
Cycle, cycles, or cyclic may refer to:
Anthropology and social sciences
* Cyclic history, a theory of history
* Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr.
* Social cycle, various cycles in s ...
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
with the chemical formula , specifically .
There are three configurational isomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms i ...
s with this structure, that differ by the arrangement of the four C–C single bonds adjacent to the double bonds. Each pair of single bonds can be on the same side () or on opposite sides () of the double bond's plane; the three possibilities are denoted , , and ; or (), (), and (). (Because of overall symmetry, is the same configuration as .)
Generally abbreviated COD, the isomer of this diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
is a useful precursor to other organic compounds and serves as a ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
in organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
. It is a colorless liquid with a strong odor. 1,5-Cyclooctadiene can be prepared by dimerization of butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vi ...
in the presence of a nickel catalyst, a coproduct being vinylcyclohexene. Approximately 10,000 tons were produced in 2005.
Organic reactions
COD reacts with borane to give 9-borabicyclo .3.1onane, commonly known as 9-BBN, a reagent in organic chemistry used inhydroboration
In organic chemistry, hydroboration refers to the addition of a hydrogen-boron bond to certain double and triple bonds involving carbon (, , , and ). This chemical reaction is useful in the organic synthesis of organic compounds.
Hydroboration p ...
s:
: COD adds (or similar reagents) to give 2,6-dichloro-9-thiabicyclo .3.1onane:
:
COD adds (or similar reagents) to give 2,6-dichloro-9-thiabicyclo .3.1onane:
: The resulting dichloride can be further modified as the di azide or di
The resulting dichloride can be further modified as the di azide or dicyano
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a ...
derivative in a nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The ...
aided by anchimeric assistance.
Metal complexes
Crabtree's catalyst
Crabtree's catalyst is an organoiridium compound with the formula 1,5-Cyclooctadiene.html" ;"title="/nowiki> C8H12IrTricyclohexylphosphine.html" ;"title="1,5-Cyclooctadiene">C8H12Ir P(C6H11)3 P(C6H11)3pyridine">C5H5N.html" ;"title="pyridine.html" ...
.
File: Cyclooctadiene-rhodium-chloride-dimer-2D-skeletal.png, The complex .
File:Co(C8H12)(C8H13).png, Co(1,5-cyclooctadiene)(cyclooctenyl).
File:PdCl2(cod).png, Dichloro(1,5‐cyclooctadiene)palladium
Dichloro(1,5-cyclooctadiene)palladium is the organopalladium compound with the formula PdCl2(C8H12) where C8H12 is cycloocta-1,5-diene (cod) or abbreviated PdCl2(cod). It is a yellow solid that is soluble in chloroform. According to X-ray crystal ...
File:Ir2Cl2 cod 2improved.svg, cyclooctadiene iridium chloride dimer
Cyclooctadiene iridium chloride dimer is an organoiridium compound with the formula r(μ2-Cl)(COD)sub>2, where COD is the diene 1,5-cyclooctadiene (C8H12). It is an orange-red solid that is soluble in organic solvents. The complex is used as a ...
File:Cl2Ptcod.png, Dichloro(1,5‐cyclooctadiene)platinum
chelate effect
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
. The COD ligands are easily displaced by other ligands, such as phosphines.
is prepared by reduction of anhydrous nickel acetylacetonate
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are tr ...
in the presence of the ligand, using triethylaluminium
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( C2H5)6 (abbreviated as Al2Et6 or TEA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially ...
:
The related is prepared by a more circuitous route involving the dilithium cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
:
:
:
Extensive work has been reported on complexes of COD, much of which has been described in volumes 25, 26, and 28 of '' Inorganic Syntheses''. The platinum complex is a precursor to a 16-electron complex of ethylene:
:
COD complexes are useful as starting materials; one noteworthy example is the reaction:
:
The product is highly toxic, thus it is advantageous to generate it in the reaction vessel upon demand. Other low-valent metal complexes of COD include cyclooctadiene rhodium chloride dimer
Cyclooctadiene rhodium chloride dimer is the organorhodium compound with the formula Rh2Cl2(C8H12)2, commonly abbreviated
, hCl(COD) HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a sp ...
or Rh2Cl2(COD)2. This yellow-orange, air-stable compound is a widely used precursor to homogeneous catalysis, h ...cyclooctadiene iridium chloride dimer
Cyclooctadiene iridium chloride dimer is an organoiridium compound with the formula r(μ2-Cl)(COD)sub>2, where COD is the diene 1,5-cyclooctadiene (C8H12). It is an orange-red solid that is soluble in organic solvents. The complex is used as a ...
, and , and Crabtree's catalyst
Crabtree's catalyst is an organoiridium compound with the formula 1,5-Cyclooctadiene.html" ;"title="/nowiki> C8H12IrTricyclohexylphosphine.html" ;"title="1,5-Cyclooctadiene">C8H12Ir P(C6H11)3 P(C6H11)3pyridine">C5H5N.html" ;"title="pyridine.html" ...
.
The complexes with nickel, palladium, and platinum have tetrahedral geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are ...
, whereas complexes of rhodium and iridium are square planar
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corne ...
.
(''E'',''E'')-COD
:George M. Whitesides
George McClelland Whitesides (born August 3, 1939) is an American chemist and professor of chemistry at Harvard University. He is best known for his work in the areas of nuclear magnetic resonance spectroscopy, organometallic chemistry, molecu ...
and Arthur C. Cope
Arthur C. Cope (June 27, 1909 – June 4, 1966) was an American organic chemist and member of the United States National Academy of Sciences. He is credited with the development of several important chemical reactions which bear his name includin ...
in 1969 by photoisomerization
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a re ...
of the ''cis'',''cis'' compound. Another synthesis (double elimination reaction from a cyclooctane ring) was reported by Rolf Huisgen
Rolf Huisgen (; 13 June 1920 – 26 March 2020) was a German chemist. His importance in synthetic organic chemistry extends to the enormous influence he had in post-war chemistry departments in Germany and Austria, due to a large number of his H ...
in 1987. The molecular conformation
A chemical structure determination includes a chemist's specifying the molecular geometry and, when feasible and necessary, the electronic structure of the target molecule or other solid. Molecular geometry refers to the spatial arrangement of at ...
of (''E'',''E'')-COD is twisted rather than chair-like. The compound has been investigated as a click chemistry mediator.
References
{{DEFAULTSORT:Cyclooctadiene, 1, 5- Cycloalkenes Dienes Ligands Eight-membered rings Foul-smelling chemicals