|
Butane-1,3-diol
1,3-Butanediol is an organic compound with the formula CH3CH(OH)CH2CH2OH, not to be confused with 1,4 Butanediol. With two alcohol functional groups, the molecule is classified as a diol. The compound without the R (or D) designation is racemic, which is what has been used in most studies before 2023. The compound is a colorless, bittersweet, water-soluble liquid. It is one of four common structural isomers of butanediol. It is used in grape flavoring, and as a precursor to some antibiotics. Production and uses Hydrogenation of 3-hydroxybutanal gives 1,3-butanediol: :CH3CH(OH)CH2CHO + H2 → CH3CH(OH)CH2CH2OH Dehydration of 1,3-butanediol gives 1,3-butadiene: :CH3CH(OH)CH2CH2OH → CH2=CH-CH=CH2 + 2H2O Pharmacology 1,3-Butanediol has sedative, hypotensive and hypoglycaemic action comparable to ethanol, with the (R), also known as (D), enantiomer being more active. Fatty acid esters of 1,3-butanediol such as the acetoacetate, lactate or hexanoate have been researched for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Butanediol
1,2-Butanediol is the organic compound with the formula HOCH(HO)CHCHCH. It is classified as a ''vic''-diol (glycol). It is chiral, although typically it is encountered as the racemic mixture. It is a colorless liquid. Preparation This diol was first described by Charles-Adolphe Wurtz in 1859. It is produced industrially by hydration of 1,2-epoxybutane.. : This process requires a ten- to twenty-fold excess of water to suppress the formation of polyethers. Depending on the amount of excess water, the selectivity varies from 70 to 92%. Sulfuric acid or strongly acidic ion exchange resins may be used as catalysts, which allows the reaction to occur under 160 °C and at slightly above atmospheric pressure. 1,2-Butanediol is a byproduct of the production of 1,4-butanediol from butadiene.. It is also a byproduct of the catalytic hydrocracking of starches and sugars such as sorbitol to ethylene glycol and propylene glycol. It can also be obtained from the dihydroxylation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,3-Butanediol
2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a ''vic''-diol (glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors to various plastics and pesticides. Isomerism Of the three stereoisomers, two are enantiomers (levo- and dextro-2,3-butanediol) and one is a meso compound. The enantiomeric pair have (2''R'', 3''R'') and (2''S'', 3''S'') configurations at carbons 2 and 3, while the meso compound has configuration (2''R'', 3''S'') or, equivalently, (2''S'', 3''R''). Industrial production and uses 2,3-Butanediol is prepared by hydrolysis of 2,3-epoxybutane:Heinz Gräfje, Wolfgang Körnig, Hans-Martin Weitz, Wolfgang Reiß, Guido Steffan, Herbert Diehl, Horst Bosche, Kurt Schneider and Heinz Kieczka "Butanediols, Butenediol, and Butynediol" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2000, Wiley-VCH, Weinheim. :(CH3CH)2O + H2O → CH3(CHOH) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is a compound that contains the same number and type of atoms, but with a different connectivity (i.e. arrangement of bonds) between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014)''Dictionary of the History of Science'' page 218. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which the atoms and bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butanediol
Butanediol, also called butylene glycol, may refer to any one of four stable structural isomers: * 1,2-Butanediol * 1,3-Butanediol * 1,4-Butanediol *2,3-Butanediol Geminal diols There are also two geminal diols (gem-diols), which are less stable: *1,1-Butanediol, hydrate of butanal *2,2-Butanediol, hydrate of butanone Isobutylene glycol and methylpropanediol Isobutylene glycol may be considered a kind of butylene glycol, similarly to butane historically including ''n''-butane and ''i''-butane (isobutane). The modern name for the closely related type of compounds is methylpropanediol. There are two stable structural isomers: *2-methylpropane-1,2-diol *2-methylpropane-1,3-diol and one unstable geminal diol: *2-methylpropane-1,1-diol (not a glycol), hydrate of 2-methylpropanal (isobutyraldehyde) These three methylpropanediols are structural isomers of butanediols. They are not chiral. Examples 2-Methylpropane-1,3-diol derivatives: * Crisnatol, an experimental medication * 2-Methy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
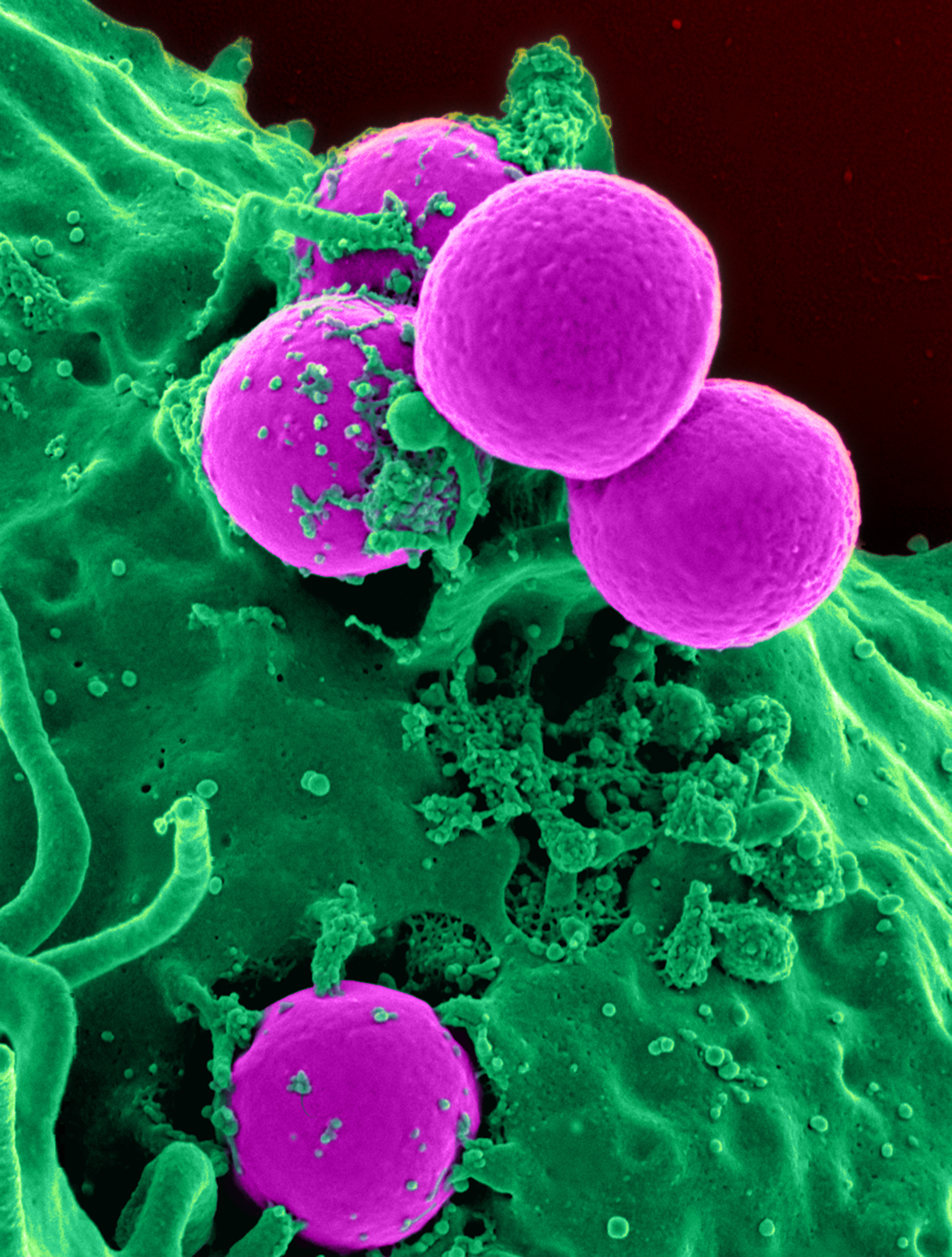
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Hydroxybutanal
In organic chemistry, 3-hydroxybutanal (acetaldol, aldol) is an organic compound with the formula and the structure . It is classified as an aldol () and the word "aldol" can refer specifically to 3-hydroxybutanal. It is formally the product of the dimerization of acetaldehyde (). A thick colorless or pale-yellow liquid, it is a versatile and valuable intermediate with diverse impacts. The compound is chiral although this aspect is not often exploited. Production Acetaldehyde dimerizes upon treatment with aqueous sodium hydroxide: : This is the prototypical aldol reaction. Reactions and uses Dehydration of 3-hydroxybutanal gives crotonaldehyde. Distillation of 3-hydroxybutanal is sufficiently forcing to effect this conversion: : Hydrogenation of 3-hydroxybutanal gives 1,3-butanediol: : This diol is a precursor to 1,3-butadiene, precursor to diverse polymers. Polymerization of 3-hydroxybutanal is also spontaneous, but can be stopped with the addition of water. Aldol has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sedative
A sedative or tranquilliser is a substance that induces sedation by reducing irritability or Psychomotor agitation, excitement. They are central nervous system (CNS) Depressant, depressants and interact with brain activity, causing its deceleration. Various kinds of sedatives can be distinguished, but the majority of them affect the neurotransmitter Gamma-Aminobutyric acid, gamma-aminobutyric acid (GABA). Most sedatives produce relaxing effects by increasing GABA activity. This group is related to hypnotics. The term ''sedative'' describes drugs that serve to calm or Anxiolytic, relieve anxiety, whereas the term ''hypnotic'' describes drugs whose main purpose is to initiate, sustain, or lengthen sleep. Because these two functions frequently overlap, and because drugs in this class generally produce dose-dependent effects (ranging from anxiolysis to loss of consciousness), they are often referred to collectively as ''sedative–hypnotic'' drugs. Terminology There is some overlap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |