|
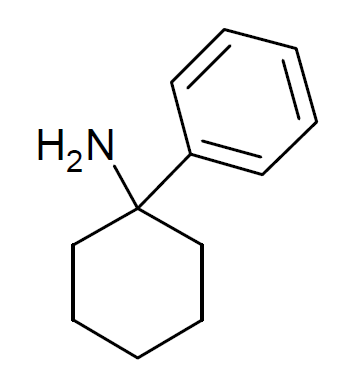
Bromadol
BDPC (systematic name 4-(4-bromophenyl)-4-(dimethylamino)-1-(2-phenylethyl)cyclohexanol; also known as bromadol) is a potent narcotic analgesic with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the strength of morphine in animal models. However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active ''trans''-isomer. This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada, and has reportedly continued to be available on the designer drug black market internationally. Analogues where the ''para''-bromine is replaced by chlorine or a methyl group retain similar activity, as does the ''meta''-hydroxyl derivative. ] ] See also * 3-OH-PCP * 4-Keto-PCP * C-8813 * Cebranopadol * Ciramadol * Dimetamine * Faxeladol * Profadol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylcyclohexylamine
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the class has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylcyclohexylamines
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the class has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faxeladol
Faxeladol (INN, USAN) (code names GRTA-9906, GRTA-0009906, EM-906, GCR-9905, GRT-TA300) is an opioid analgesic which was developed by Grünenthal GmbH but was never marketed for medical use anywhere in the world. It is related to tramadol and ciramadol, and was developed shortly after tramadol in the late 1970s. Similarly to tramadol, it was believed faxeladol would have analgesic, as well as antidepressant effects, due to its action on serotonin and norepinephrine reuptake. In various studies in the 1970s alongside tramadol, faxeladol was seen to be slightly more potent than tramadol, but with a higher rate of sudden seizures than tramadol, which is known to cause seizures without warning in some users. See also * Bromadol * Profadol * Tapentadol Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-8813
C-8813 (thiobromadol) is a potent μ-opioid receptor agonist with a distinctive chemical structure which is not closely related to other established families of opioid drugs. The ''trans''-isomer was found to be around 591 times more potent than morphine in animal studies. The same study assigned a potency of 504 times that of morphine to the related compound BDPC. C-8813 is claimed to be similarly potent at the δ-opioid receptor, which antagonizes the mu depression of breathing, presumably making the drug safer. C-8813 has never been used in humans. See also * BDPC * Ciramadol * Faxeladol * Profadol * Tapentadol * Tramadol Tramadol, sold under the brand name Ultram among others, is an opioid pain medication used to treat moderate to moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an h ... References Arylcyclohexylamines Synthetic opioids Thiophenes Tertiary alcohols Organobrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Keto-PCP
4-Keto-PCP is a recreational designer drug from the arylcyclohexylamine family, with dissociative effects. It has potency in between that of ketamine and phencyclidine but with somewhat more sedating effects in animal studies. See also * 3-HO-PCP * 3-Fluoro-PCP * Bromadol * Dimetamine * Methoxetamine Methoxetamine, abbreviated as MXE, is a dissociative hallucinogen that has been sold as a designer drug. It differs from many dissociatives such as ketamine and phencyclidine (PCP) that were developed as pharmaceutical drugs for use as general ... References Arylcyclohexylamines Designer drugs Dissociative drugs 1-Piperidinyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamino Compounds
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tramadol
Tramadol, sold under the brand name Ultram among others, is an opioid pain medication used to treat moderate to moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen). As is typical of opioids, common side effects include constipation, itchiness, and nausea. Serious side effects may include hallucinations, seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction. A change in dosage may be recommended in those with kidney or liver problems. It is not recommended in those who are at risk of suicide or in those who are pregnant. While not recommended in women who are breastfeeding, those who take a single dose should not generally stop breastfeeding. Tramadol is converted in the liver to ''O''-desmethyltramadol (desmetramadol), an opioid with a stronger affinity to the μ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tapentadol
Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration, and lasts for 4–6 hours. It is similar to tramadol in its dual mechanism of action; namely, its ability to activate the mu opioid receptor and inhibit the reuptake of norepinephrine. Unlike tramadol, it has only weak effects on the reuptake of serotonin and is a significantly more potent opioid with no known active metabolites. Tapentadol is not a pro-drug and therefore does not rely on metabolism to produce its therapeutic effects; this makes it a useful moderate-potency analgesic option for patients who do not respond adequately to more commonly used opioids due to genetic disposition (poor metabolizers of CYP3A4 and CYP2D6), as well as providing a more consistent dosage-response range among the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Profadol
Profadol (CI-572) is an opioid analgesic which was developed in the 1960s by Parke-Davis. It acts as a mixed agonist-antagonist of the μ-opioid receptor. The analgetic potency is about the same as of pethidine (meperidine), the antagonistic effect is 1/50 of nalorphine. Synthesis The Knoevenagel condensation between 3'-Methoxybutyrophenone 1550-06-1and Ethyl cyanoacetate gives (1). Conjugate addition of cyanide gives (2). Hydrolysis of both nitrile groups, saponification of the ester and decarboxylation gives the diacidCID:164137621(3). Imide formation occurs upon treatment with methylamine giving 3-(3-Methoxyphenyl)-1-methyl-3-propylpyrrolidine-2,5-dioneCID:163444474(4). Reduction of the imide by lithium aluminium hydride gave 505-32-429369-01-5] (5). Demethylation completed the synthesis of Profadol (6). See also * BDPC, Bromadol * C-8813 * Ciramadol * Faxeladol * Prodilidine * Tapentadol * Tramadol Tramadol, sold under the brand name Ultram among others, is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Dimethylamino-4-(p-tolyl)cyclohexanone
4-Dimethylamino-4-(''p''-tolyl)cyclohexanone (sometimes known as dimetamine) is a narcotic analgesic with an arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. It has around the same analgesic potency as morphine, with analogues where the ''p''-methyl group is replaced by chlorine or bromine being slightly weaker. However derivatives where the ketone group has been reacted with a Grignard reagent to add a phenethyl substitution are several hundred times stronger, and in this series it is the bromo compound BDPC that is the most potent. Legal Status 4-Dimethylamino-4-(p-tolyl)cyclohexanone is specifically listed as an illegal drug in Latvia. It is also covered by drug analogue laws in various jurisdictions as a generic arylcyclohexylamine derivative. See also * 3-HO-PCP * 4-Keto-PCP * Tramadol Tramadol, sold under the brand name Ultram among others, is an opioid pain medication used to treat moderate to moderately severe pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |