|
4-Dimethylamino-4-(p-tolyl)cyclohexanone
4-Dimethylamino-4-(''p''-tolyl)cyclohexanone (sometimes known as dimetamine) is a narcotic analgesic with an arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. It has around the same analgesic potency as morphine, with analogues where the ''p''-methyl group is replaced by chlorine or bromine being slightly weaker. However derivatives where the ketone group has been reacted with a Grignard reagent to add a phenethyl substitution are several hundred times stronger, and in this series it is the bromo compound BDPC that is the most potent. Legal Status 4-Dimethylamino-4-(p-tolyl)cyclohexanone is specifically listed as an illegal drug in Latvia. It is also covered by drug analogue laws in various jurisdictions as a generic arylcyclohexylamine derivative. See also * 3-HO-PCP * 4-Keto-PCP * Tramadol Tramadol, sold under the brand name Ultram among others, is an opioid pain medication used to treat moderate to moderately severe pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BDPC
BDPC (systematic name 4-(4-bromophenyl)-4-(dimethylamino)-1-(2-phenylethyl)cyclohexanol; also known as bromadol) is a potent narcotic analgesic with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the strength of morphine in animal models. However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active ''trans''-isomer. This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada, and has reportedly continued to be available on the designer drug black market internationally. Analogues where the ''para''-bromine is replaced by chlorine or a methyl group retain similar activity, as does the ''meta''-hydroxyl derivative. ] ] See also * 3-OH-PCP * 4-Keto-PCP * C-8813 * Cebranopadol * Ciramadol * Dimetamine * Faxeladol * Profadol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
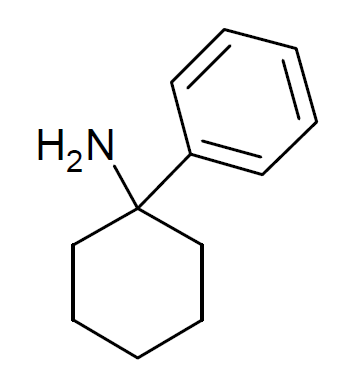
Arylcyclohexylamine
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the class has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylcyclohexylamines
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the class has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Keto-PCP
4-Keto-PCP is a recreational designer drug from the arylcyclohexylamine family, with dissociative effects. It has potency in between that of ketamine and phencyclidine but with somewhat more sedating effects in animal studies. See also * 3-HO-PCP * 3-Fluoro-PCP * Bromadol * Dimetamine * Methoxetamine Methoxetamine, abbreviated as MXE, is a dissociative hallucinogen that has been sold as a designer drug. It differs from many dissociatives such as ketamine and phencyclidine (PCP) that were developed as pharmaceutical drugs for use as general ... References Arylcyclohexylamines Designer drugs Dissociative drugs 1-Piperidinyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-HO-PCP
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug. Pharmacology 3-HO-PCP acts as a high-affinity uncompetitive antagonist of the NMDA receptor via the dizocilpine (MK-801) site (Ki = 30 nM). It has much higher affinity than PCP for this site (Ki = 250 nM, for comparison; 8-fold difference). The drug also has high affinity for the μ-opioid receptor (MOR) (Ki = 39–60 nM) in animal test subjects, the κ-opioid receptor (KOR) (Ki = 140 nM), and the sigma σ1 receptor (Ki = 42 nM; IC50 = 19 nM), whereas it has only low affinity for the δ-opioid receptor (Ki = 2,300 nM). The high affinity of 3-HO-PCP for opioid receptors is unique among arylcyclohexylamines and is in contrast to PCP, which has only very low affinity for the MOR (Ki = 11,000–26,000 nM; 282- to 433-fold difference) and the other opioid receptors (Ki = 4,100 n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylhexedrine
Propylhexedrine, sold under the brand name Benzedrex, is a nasal decongestant, appetite suppressant, and psychostimulant medication. It is used medicinally for relief of congestion due to colds, allergies and allergic rhinitis. Propylhexedrine is most commonly found in over-the-counter Benzedrex inhalers. Benzedrex was first manufactured by Smith, Kline and French after the Benzedrine inhaler, which contained racemic amphetamine, became unavailable following the placement of amphetamines on the US Schedule II status (highest abuse potential, yet with accepted medicinal uses). Benzedrex is currently manufactured by B.F. Ascher & Co. Inc. Pharmaceuticals. Propylhexedrine has also seen use in Europe as an appetite suppressant, under the trade name Obesin. Additionally, it is found in the anticonvulsant preparation barbexaclone, where its ''S''-isomer ( levopropylhexedrine or L-propylhexedrine) is bonded with phenobarbital for the purpose of offsetting the barbiturate-induced s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamino Compounds
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tramadol
Tramadol, sold under the brand name Ultram among others, is an opioid pain medication used to treat moderate to moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen). As is typical of opioids, common side effects include constipation, itchiness, and nausea. Serious side effects may include hallucinations, seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction. A change in dosage may be recommended in those with kidney or liver problems. It is not recommended in those who are at risk of suicide or in those who are pregnant. While not recommended in women who are breastfeeding, those who take a single dose should not generally stop breastfeeding. Tramadol is converted in the liver to ''O''-desmethyltramadol (desmetramadol), an opioid with a stronger affinity to the μ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Medicinal Chemistry
A journal, from the Old French ''journal'' (meaning "daily"), may refer to: *Bullet journal, a method of personal organization *Diary, a record of what happened over the course of a day or other period *Daybook, also known as a general journal, a daily record of financial transactions *Logbook, a record of events important to the operation of a vehicle, facility, or otherwise *Record (other) *Transaction log, a chronological record of data processing *Travel journal In publishing, ''journal'' can refer to various periodicals or serials: *Academic journal, an academic or scholarly periodical **Scientific journal, an academic journal focusing on science **Medical journal, an academic journal focusing on medicine **Law review, a professional journal focusing on legal interpretation *Magazine, non-academic or scholarly periodicals in general **Trade magazine, a magazine of interest to those of a particular profession or trade **Literary magazine, a magazine devoted to literat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek (bromos) meaning "stench", referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a native element in nature but it occurs in colourless soluble crystalline mineral halide salts, analogous to table salt. In fact, bromine and all the halogens are so reactive that they form bonds in pairs—never in single atoms. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its accumulation in the oceans. Commercial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Narcotic
The term narcotic (, from ancient Greek ναρκῶ ''narkō'', "to make numb") originally referred medically to any psychoactive compound with numbing or paralyzing properties. In the United States, it has since become associated with opiates and opioids, commonly morphine and heroin, as well as derivatives of many of the compounds found within raw opium latex. The primary three are morphine, codeine, and thebaine (while thebaine itself is only very mildly psychoactive, it is a crucial precursor in the vast majority of semi-synthetic opioids, such as oxycodone or hydrocodone). Legally speaking, the term "narcotic" may be imprecisely defined and typically has negative connotations. When used in a legal context in the U.S., a narcotic drug is totally prohibited, such as heroin, or one that is used in violation of legal regulation (in this word sense, equal to any controlled substance or illicit drug). In the medical community, the term is more precisely defined and genera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |