|
Antonín Holý
Antonín Holý (1 September 1936 – 16 July 2012) was a pioneering Czech scientist. He specialised in the field of chemistry and cooperated on the development of important antiretroviral drugs used in the treatment of HIV and hepatitis B. He was involved in the creation of the most effective drug (as of early 2009) in the treatment of AIDS. Antonín Holý is the author of more than 450 papers, 400 scientific discoveries and holds 60 patents. With more than 400 discoveries to his credit, his work has affected millions of people with viral diseases such as HIV/AIDS and hepatitis B and many other viral diseases. In 2008 he received an Honorary Professorship at the University of Manchester's School of Chemistry. Scientific career Born in Prague, Czechoslovakia, Antonín Holý studied organic chemistry from 1954 to 1959 at the Faculty of Science of Charles University in Prague. From 1960 he trained at the Institute of Organic Chemistry and Biochemistry (IOCB) of the Czechoslovak Acad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prague
Prague ( ; cs, Praha ; german: Prag, ; la, Praga) is the capital and largest city in the Czech Republic, and the historical capital of Bohemia. On the Vltava river, Prague is home to about 1.3 million people. The city has a temperate oceanic climate, with relatively warm summers and chilly winters. Prague is a political, cultural, and economic hub of central Europe, with a rich history and Romanesque, Gothic, Renaissance and Baroque architectures. It was the capital of the Kingdom of Bohemia and residence of several Holy Roman Emperors, most notably Charles IV (r. 1346–1378). It was an important city to the Habsburg monarchy and Austro-Hungarian Empire. The city played major roles in the Bohemian and the Protestant Reformations, the Thirty Years' War and in 20th-century history as the capital of Czechoslovakia between the World Wars and the post-war Communist era. Prague is home to a number of well-known cultural attractions, many of which survived the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rega Institute For Medical Research
The Rega Institute for Medical Research is a Belgian scientific establishment that is part of the Catholic University of Leuven (Leuven) in central Belgium. The Rega Institute is an interfacultary biomedical research institute of the Catholic University of Leuven and consists of departments of medicine and pharmacology. Divisions * Molecular Bacteriology * Clinical and Epidemiological Virology * Immunobiology * Medicinal Chemistry * Molecular Immunology * Virology and Chemotherapy History The Rega Institute was founded in 1954 within the unitary Catholic University of Leuven by Pieter De Somer, who named it for Henri-Joseph Rega, a Professor at the Old University of Leuven in the 18th century. The building of the Rega Institute was constructed in 1954 and paid by the company Recherche et Industrie Thérapeutiques (R.I.T.) in Rixensart, where Dr. De Somer had a leading function. During the sixties, De Somer withdrew from R.I.T. and in 1968 he became the founder and first rector ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Czech News Agency
The Czech News Agency ( cs, Česká tisková kancelář), abbreviated to ČTK, is a national public service news agency in the Czech Republic. It publishes in Czech and English. It discontinued its Slovak language service on 1 January 2011. Founded on 28 October 1918, on the same day as Czechoslovakia's formation, the company has been owned by the government and used by the various regimes in the Czech lands since then. Following the Velvet Revolution of 1989, the government ceased interfering in editorial decisions. In 1993 the government relinquished control of the agency, which has since been governed by a board of seven people elected by the lower house of Parliament. Members of the board are not allowed to be politically active. The agency's state subsidy was discontinued in 1996. It was renamed from Czechoslovak to Czech News Agency on 1 January 1993 when Czechoslovakia split. CTK, however, stayed active in the Slovak market. Its former Slovak part is a separate company ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tomáš Cihlář
Tomáš Cihlář (born 1967) is a Czech biochemist known for his role in the development of remdesivir. A specialist in virology, Cihlář holds the positions of Senior Director, Biology, and Vice-President at American pharmaceutical company Gilead Sciences. As a student, Cihlář assisted fellow biochemist Antonín Holý in developing Viread, the primary drug used to fight HIV infection. Education Cihlář graduated from the University of Chemistry and Technology, Prague, majoring in fermentation chemistry and bioengineering under the tutelage of Jan Páca and Vladimír Jirků. He completed his postgraduate studies at the Institute of Organic Chemistry and Biochemistry of the ASCR under Ivan Rosenberg and Ivan Votruba. In 1994 he obtained the title of Candidate of Sciences and was engaged in the research of antiviral agents under the tutelage of Antonín Holý. That same year, he traveled to the United States for a postdoctoral fellowship with Gilead Sciences, where as researche ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilead Sciences
Gilead Sciences, Inc. () is an American biopharmaceutical company headquartered in Foster City, California, that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500. Gilead was founded in 1987 under the name Oligogen by Michael L. Riordan. The original name was a reference to oligomers, small strands of DNA used to target genetic sequences. Gilead held its IPO in 1992, and successfully developed drugs like Tamiflu and Vistide that decade. In the 2000s, Gilead received approval for drugs including Viread and Hepsera, among others. It began evolving from a biotechnology company into a pharmaceutical company, acquiring several subsidiaries, though it still relied heavily on contracting to manufacture its drugs. The company continued its growth in the 2010s, but came under heavy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopharmaceutical
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine. Terminology surrounding biopharmaceuticals varies between groups and entities, with different terms referring to different subsets of therapeutics within the general biopharmaceutical category. Some regulatory agencies use the terms ''biological ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepsera
Adefovir is a prescription medicine used to treat (chronic) infections with hepatitis B virus. A prodrug form of adefovir was previously called bis-POM PMEA, with trade names Preveon and Hepsera. It is an orally administered nucleotide analog reverse-transcriptase inhibitor (ntRTI). It can be formulated as the pivoxil prodrug adefovir dipivoxil. Uses It is used for treatment of hepatitis B. Trials of adefovir in patients with HIV have not shown any clear benefits. History Adefovir was invented in the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic by Antonín Holý, and the drug was developed by Gilead Sciences for HIV with the brand name Preveon. However, in November 1999, an expert panel advised the U.S. Food and Drug Administration (FDA) not to approve the drug due to concerns about the severity and frequency of kidney toxicity when dosed at 60 or 120 mg. The FDA followed that advice, refusing to approve adefovir as a treatment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pivaloyloxymethyl
Pivaloyloxymethyl (POM, pivoxil, pivoxyl) is a protecting group used in organic synthesis.''Protective Groups in Organic Synthesis'', 3rd Edition, Theodora M. Greene and Peter G. M. Wuts, The POM radical has the formula (CH3)3C-CO-O-CH2. The POM group is also sometimes used to produce prodrugs. For example, the POM group can be attached to a negatively charged organophosphate group(s) of a drug causing a neutralization of the negative charge, which can allow the drug to become more lipid-soluble and thus more able to diffuse passively across cell membranes into cells. Upon entry into cells, the POM portion of the molecule can be removed by cellular processes resulting in release of active drug. Clinically used prodrugs containing pivaloyloxymethyl groups include adefovir dipivoxil, pivampicillin, cefditoren pivoxil, pivmecillinam, and valproate pivoxil. Tenofovir disoproxil contains a very similar prodrug group. See also * Pivalic acid Pivalic acid is a carboxylic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cidofovir
Cidofovir, brand name Vistide, is a topical or injectable antiviral medication primarily used as a treatment for cytomegalovirus (CMV) retinitis (an infection of the retina of the eye) in people with AIDS. Cidofovir was approved for medical use in 1996. Medical use DNA virus Its only indication that has received regulatory approval worldwide is cytomegalovirus retinitis. Cidofovir has also shown efficacy in the treatment of aciclovir-resistant HSV infections. Cidofovir has also been investigated as a treatment for progressive multifocal leukoencephalopathy with successful case reports of its use. Despite this, the drug failed to demonstrate any efficacy in controlled studies. Cidofovir might have anti-smallpox efficacy and might be used on a limited basis in the event of a bioterror incident involving smallpox cases. Brincidofovir, a cidofovir derivative with much higher activity against smallpox that can be taken orally has been developed. It has inhibitory effects on var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
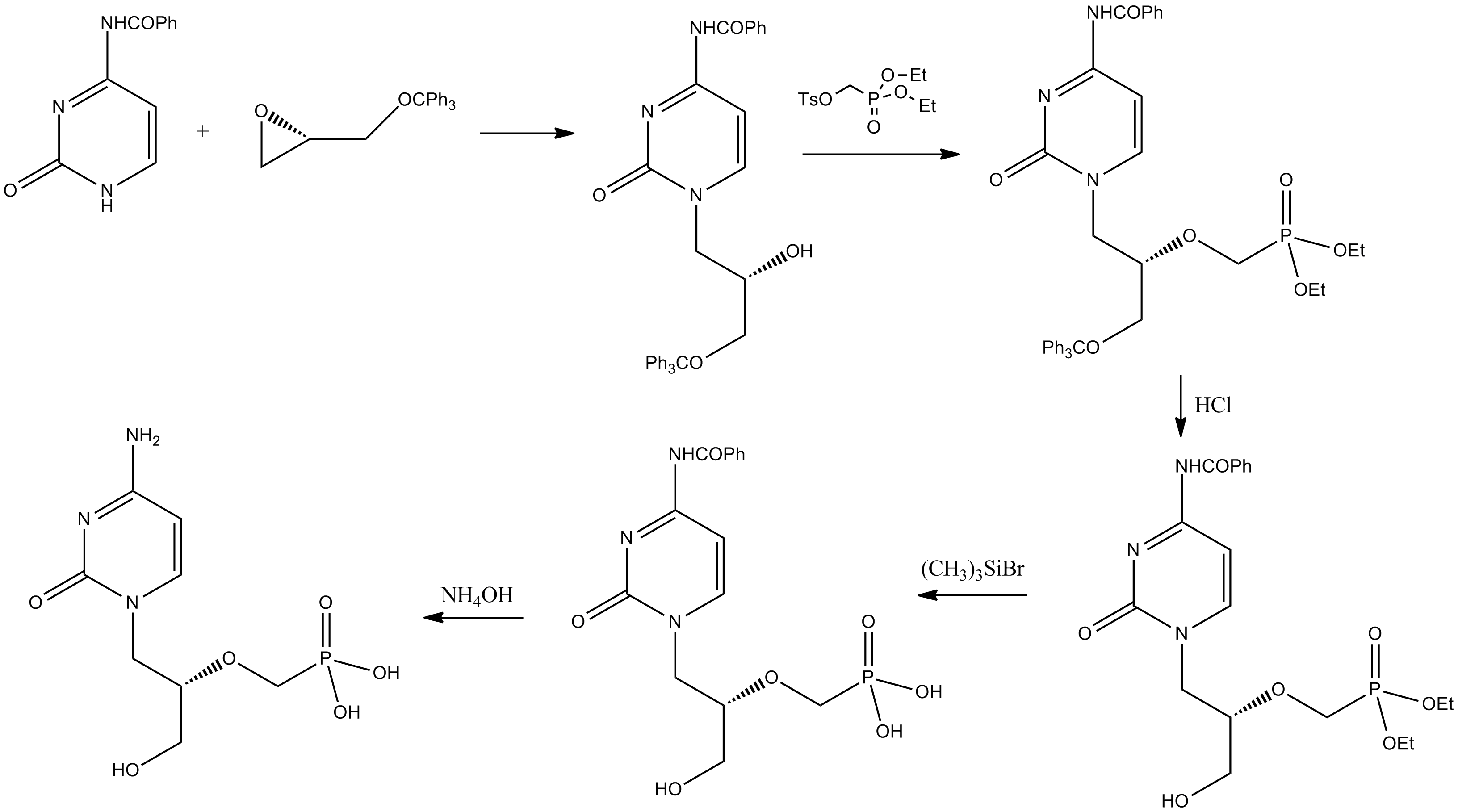
Adefovir
Adefovir is a prescription medicine used to treat (chronic) infections with hepatitis B virus. A prodrug form of adefovir was previously called bis-POM PMEA, with trade names Preveon and Hepsera. It is an orally administered nucleotide analog reverse-transcriptase inhibitor (ntRTI). It can be formulated as the pivoxil prodrug adefovir dipivoxil. Uses It is used for treatment of hepatitis B. Trials of adefovir in patients with HIV have not shown any clear benefits. History Adefovir was invented in the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic by Antonín Holý, and the drug was developed by Gilead Sciences for HIV with the brand name Preveon. However, in November 1999, an expert panel advised the U.S. Food and Drug Administration (FDA) not to approve the drug due to concerns about the severity and frequency of kidney toxicity when dosed at 60 or 120 mg. The FDA followed that advice, refusing to approve adefovir as a treatment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tenofovir
Tenofovir disoproxil, sold under the trade name Viread among others, is a medication used to treat chronic hepatitis B and to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention of HIV/AIDS among those at high risk before exposure, and after a needlestick injury or other potential exposure. It is sold both by itself and together in combinations such as emtricitabine/tenofovir, efavirenz/emtricitabine/tenofovir, and elvitegravir/cobicistat/emtricitabine/tenofovir. It does not cure HIV/AIDS or hepatitis B. It is available by mouth as a tablet or powder. Common side effects include nausea, rash, diarrhea, headache, pain, depression, and weakness. Severe side effects include lactic acidosis, high blood lactate and an hepatomegaly, enlarged liver. There are no absolute contraindications. It is often recommended during pregnancy and appears to be safe. It is a Reverse-transcriptase inhibitor, nucleotide reverse tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |