|
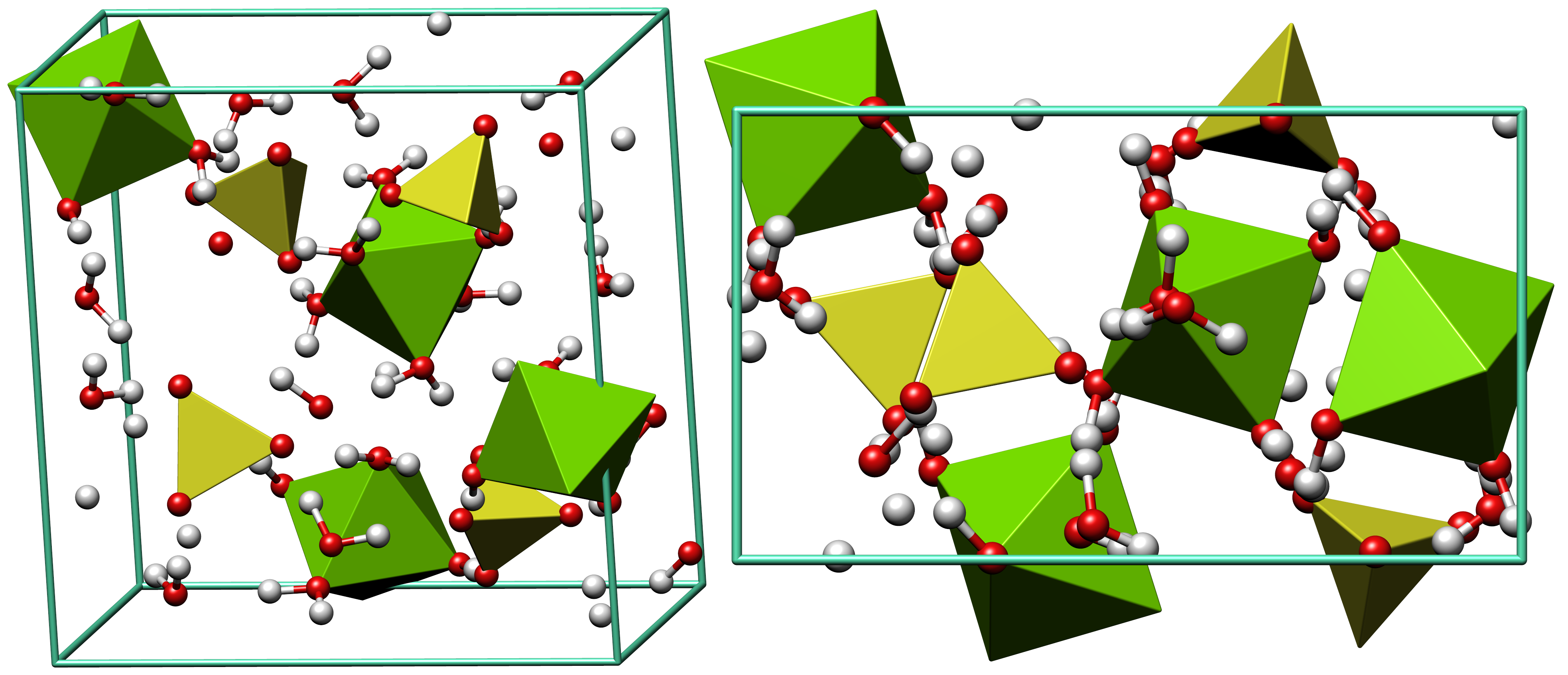
Alunogen
Alunogen (from French ''alun'', “alum”), also called feather alum and hair salt is a colourless to white (although often coloured by impurities, such as iron substituting for aluminium) fibrous to needle-like aluminium sulfate mineral. It has the chemical formula Al2(SO4)3·17H2O. It is often found on the walls of mines and quarries as a secondary mineral. It can be found in the oxidation zones of some ore deposits as well as on burning coal dumps (i.e., as the product of millosevichite hydration). It also forms as a low temperature deposit in fumaroles. It occurs associated with pyrite, marcasite, halotrichite, pickeringite, epsomite, potash alum, melanterite and gypsum Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. .... The crystallochemical formula, can be written as: l(H2O) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 ** Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O ** Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite Cu3SO4(OH)4 **Brochantite Cu4SO4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Minerals
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal Vein (geology), veins and as secondary minerals in the Redox, oxidizing zone of sulfide mineral deposits. The Chromate ion, chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 **Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O **Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 ** Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O ** Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite Cu3SO4(OH)4 **Brochantite Cu4SO4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pickeringite
Pickeringite is a magnesium aluminium sulfate mineral with formula MgAl2(SO4)4·22(H2O). It forms a series with halotrichite. It forms as an alteration product of pyrite in aluminium rich rocks and in coal seams. It also occurs in pyrite rich hydrothermal ore deposits in arid regions. It forms in fumaroles and in caves. It occurs with kalinite Kalinite is a mineral composed of hydrated potassium aluminium sulfate (a type of alum). It is a fibrous monoclinic alum, distinct from isometric potassium alum,American Mineralogist (1923) 8:15 named in 1868. Its name comes from ''kalium'' ..., alunogen, epsomite, melanterite, copiapite and gypsum. It was first described in 1844 for an occurrence in Cerros Pintados, Pampa del Tamarugal, Iquique Province, Tarapacá Region, Chile. It was named for American linguist and philologist John Pickering (linguist), John Pickering (1777–1846). References Sulfate minerals Monoclinic minerals Minerals in space group 14 {{sulfate-miner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Minerals
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity towards oxyg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywall. Alabaster, a fine-grained white or lightly tinted variety of gypsum, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison. Etymology and history The word ''gypsum'' is derived from the Greek word (), "plaster". Because the quarries of the Montmartre district of Paris have long furnished burnt gypsum (calcined gypsum) used for various purposes, this dehydrated gypsum became known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanterite
Melanterite is a mineral form of hydrous iron(II) sulfate: FeSO4·7H2O. It is the iron analogue of the copper sulfate chalcanthite. It alters to siderotil by loss of water. It is a secondary sulfate mineral which forms from the oxidation of primary sulfide minerals such as pyrite and marcasite in the near-surface environment. It often occurs as a ''post mine'' encrustation on old underground mine surfaces. It also occurs in coal and lignite seams exposed to humid air and as a rare sublimate phase around volcanic fumaroles. Associated minerals include pisanite, chalcanthite, epsomite, pickeringite, halotrichite Halotrichite, also known as ''feather alum'', is a highly hydrated sulfate of aluminium and iron. Its chemical formula is FeAl2(SO4)4·22H2O. It forms fibrous monoclinic crystals. The crystals are water-soluble. It is formed by the weathering and ... and other sulfate minerals. It was first described in 1850. Gallery File:Melanterite crystal structure.png, Crystal str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potash Alum
Potassium alum, potash alum, or potassium aluminium sulfate is a chemical compound: the double sulfate of potassium and aluminium, with chemical formula KAl(SO4)2. It is commonly encountered as the dodecahydrate, KAl(SO4)2·12H2O. It crystallizes in an octahedral structure in neutral solution and cubic structure in an alkali solution with space group P a −3 and lattice parameter of 12.18 Å. The compound is the most important member of the generic class of compounds called alums, and is often called simply alum. Potassium alum is commonly used in water purification, leather tanning, dyeing, fireproof textiles, and baking powder as E number E522. It also has cosmetic uses as a deodorant, as an aftershave treatment and as a styptic for minor bleeding from shaving.Alum Block for Shaving � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epsomite
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula MgSO4·7H2O. Epsomite crystallizes in the orthorhombic system as rarely found acicular or fibrous crystals, the normal form is as massive encrustations. It is colorless to white with tints of yellow, green and pink. The Mohs hardness is 2 to 2.5 and it has a low specific gravity of 1.67. It is readily soluble in water. It absorbs water from the air and converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. Discovery and occurrence Epsomite forms as encrustations or efflorescences on limestone cavern walls and mine timbers and walls, rarely as volcanic fumarole deposits, and as rare beds in evaporite layers such as those found in certain bodies of salt water. It occurs in association with melanterite, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marcasite
The mineral marcasite, sometimes called “white iron pyrite”, is iron sulfide (FeS2) with orthorhombic crystal structure. It is physically and crystallographically distinct from pyrite, which is iron sulfide with cubic crystal structure. Both structures do have in common that they contain the disulfide S22− ion, having a short bonding distance between the sulfur atoms. The structures differ in how these di-anions are arranged around the Fe2+ cations. Marcasite is lighter and more brittle than pyrite. Specimens of marcasite often crumble and break up due to the unstable crystal structure. On fresh surfaces, it is pale yellow to almost white and has a bright metallic luster. It tarnishes to a yellowish or brownish color and gives a black streak. It is a brittle material that cannot be scratched with a knife. The thin, flat, tabular crystals, when joined in groups, are called “cockscombs”. In marcasite jewellery, pyrite used as a gemstone is called “marcasite” – t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halotrichite
Halotrichite, also known as ''feather alum'', is a highly hydrated sulfate of aluminium and iron. Its chemical formula is FeAl2(SO4)4·22H2O. It forms fibrous monoclinic crystals. The crystals are water-soluble. It is formed by the weathering and decomposition of pyrite commonly near or in volcanic vents. The locations of natural occurrences include: the Atacama Desert, Chile; Dresden in Saxony, Germany; San Juan County, Utah; Iceland and Mont Saint-Hilaire, Canada. The name is from Latin: ''halotrichum'' for salt hair which accurately describes the precipitate/evaporite mineral. ;Gallery File:Halotrichite Hydrous iron aluminum sulfate Corral, California 3009.jpg, Halotrichite from California File:Halotrichite-179634.jpg, Halotrichite from the abandoned Golden Queen mine on Soledad Mountain south of Mojave, California Mojave (formerly Mohave) is an Unincorporated area, unincorporated community in Kern County, California, United States. Mojave is located east of Bakersfield, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triclinic
180px, Triclinic (a ≠ b ≠ c and α ≠ β ≠ γ ) In crystallography, the triclinic (or anorthic) crystal system is one of the 7 crystal systems. A crystal system is described by three basis vectors. In the triclinic system, the crystal is described by vectors of unequal length, as in the orthorhombic system. In addition, the angles between these vectors must all be different and may not include 90°. The triclinic lattice is the least symmetric of the 14 three-dimensional Bravais lattices. It has (itself) the minimum symmetry all lattices have: points of inversion at each lattice point and at 7 more points for each lattice point: at the midpoints of the edges and the faces, and at the center points. It is the only lattice type that itself has no mirror planes. Crystal classes The triclinic crystal system class names, examples, Schönflies notation, Hermann-Mauguin notation, point groups, International Tables for Crystallography space group number, orbifold, type, and sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |