|
Arsenic Pentafluoride
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. It is a toxic, colorless gas. The oxidation state of arsenic is +5. Synthesis Arsenic pentafluoride can be prepared by direct combination of arsenic and fluorine: :2As + 5F2 → 2AsF5 It can also be prepared by the reaction of arsenic trifluoride and fluorine: :AsF3 + F2 → AsF5 or the addition of fluorine to arsenic pentoxide or arsenic trioxide. :2As2O5 + 10F2 → 4AsF5 + 5O2 :2As2O3 + 10F2 → 4AsF5 + 3O2 Properties Arsenic pentafluoride is a colourless gas and has a trigonal bipyramidal structure. In the solid state the axial As−F bond lengths are 171.9 pm and the equatorial 166.8 pm. Its point group is D3h. Reactions Arsenic pentafluoride forms halide complexes and is a powerful fluoride acceptor. An example is the reaction with sulfur tetrafluoride, forming an ionic hexafluoroarsenate complex.An investigation of the structures of the adducts of SF4 with BF3, PF5, AsF5, and SbF5 in the solid sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation State
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge. The oxidation state of an atom does not represent the "real" formal charge on that atom, or any other actual atomic property. This is particularly true of high oxidation states, where the ionization energy required to produce a multiply positive ion is far greater than the energies available in chemical reactions. Additionally, the oxidation states of atoms in a given compound may vary depending on the choice of electronegativity scale used in their calculation. Thus, the oxidation state of an atom in a compound is purely a formalism. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic(V) Compounds
Arsenic is a chemical element with the symbol As and atomic number 33. Arsenic occurs in many minerals, usually in combination with sulfur and metals, but also as a pure elemental crystal. Arsenic is a metalloid. It has various allotropes, but only the gray form, which has a metallic appearance, is important to industry. The primary use of arsenic is in alloys of lead (for example, in car batteries and ammunition). Arsenic is a common n-type dopant in semiconductor electronic devices. It is also a component of the III-V compound semiconductor gallium arsenide. Arsenic and its compounds, especially the trioxide, are used in the production of pesticides, treated wood products, herbicides, and insecticides. These applications are declining with the increasing recognition of the toxicity of arsenic and its compounds. A few species of bacteria are able to use arsenic compounds as respiratory metabolites. Trace quantities of arsenic are an essential dietary element in rats, hamsters, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Highly Toxic Gases
Many gases have toxic properties, which are often assessed using the LC50 (median lethal dose) measure. In the United States, many of these gases have been assigned an NFPA 704 health rating of 4 (may be fatal) or 3 (may cause serious or permanent injury), and/or exposure limits ( TLV, TWA or STEL) determined by the ACGIH professional association. Some, but by no means all, toxic gases are detectable by odor, which can serve as a warning. Among the best known toxic gases are carbon monoxide, chlorine, nitrogen dioxide and phosgene. Definition *Toxic: it is a chemical that has a median lethal concentration (LC50) in air of more than 200 parts per million (ppm) but not more than 2,000 parts per million by volume of gas or vapor, or more than 2 milligrams per liter but not more than 20 milligrams per liter of mist, fume or dust, when administered by continuous inhalation for 1 hour (or less if death occurs within 1 hour) to albino rats weighing between 200 and 300 grams each. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). About 13 million metric tonne are produced annually. VCM is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States currently remains the largest VCM manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of VCM. Vinyl chloride is a gas with a sweet odor. It is highly toxic, flammable, and carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluoroarsenate
The hexafluoroarsenate (sometimes shortened to fluoroarsenate) anion is a chemical species with formula . Hexafluoroarsenate is relatively inert, being the conjugate base of the notional superacid hexafluoroarsenic acid (). Synthesis The first undisputed synthesis is due to Otto Ruff, Kurt Stäuber and Hugo Graf, who began with the lower-valent arsenic trifluoride, using silver(I) fluoride as both a fluorine source and oxidant: cites but discounts it as describing an implausibly easy synthesis with a hydrolyzable product. AsF3 + 3AgF + NOCl -> NOAsF6 + AgCl + 2AgIn the following reaction, one mole of arsenic trifluoride, three moles of silver fluoride, and one mole of nitrosyl chloride to produce one mole of nitrosyl hexafluoroarsenate, one mole of silver chloride, and two moles of elemental silver. Modern syntheses usually begin with arsenic pentafluoride (), which abstracts fluoride from common donors, such as hydrogen fluoride () or ''cis''- difluorodiazine (). Alt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the formal +4 oxidation state. Of sulfur's total of six valence electrons, two form a lone pair. The structure of SF4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. In c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
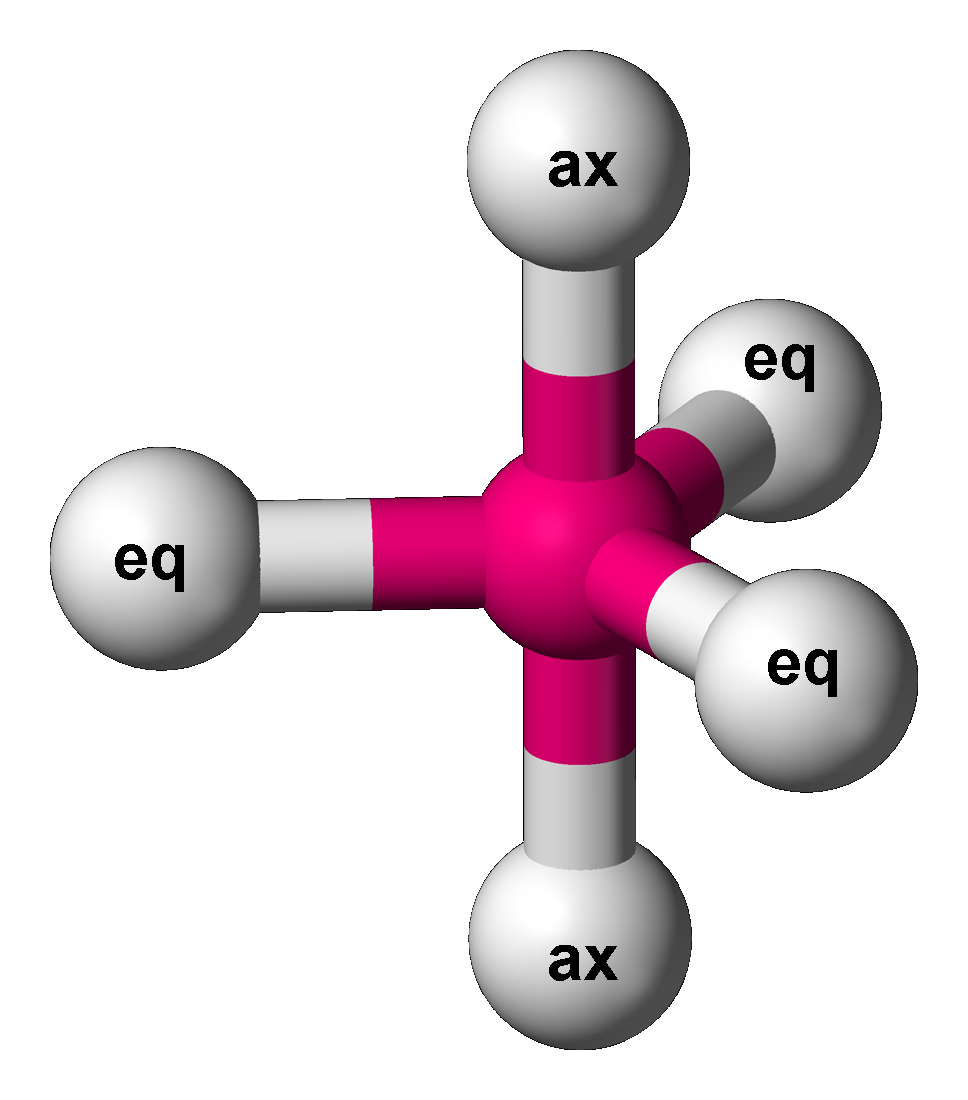
Trigonal Bipyramidal
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crowd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Trioxide
Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound and medication. As an industrial chemical, whose major uses include in the manufacture of wood preservatives, pesticides, and glass. As a medication, it is used to treat a type of cancer known as acute promyelocytic leukemia. For this use it is given by injection into a vein. Common side effects include vomiting, diarrhea, swelling, shortness of breath, and headaches. Severe side effects may include APL differentiation syndrome and heart problems. Use during pregnancy or breastfeeding may harm the baby. Arsenic trioxide has the formula . Its mechanism in treating cancer is not entirely clear. Arsenic trioxide was approved for medical use in the United States in 2000. It is on the World Health Organization's List of Essential Medicines. Approximately 50,000 tonnes are produced a year. Due to its toxicity, a number of countries have regulations around its manufacture and sale. Uses M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Pentoxide
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications. Structure The structure consists of tetrahedral and octahedral centers linked by sharing corners. The structure differs from that of the corresponding phosphorus(V) oxide; as a result, although there is still a solid solution with that oxide, it only progresses to the equimolar point, at which point phosphorus has substituted for arsenic in all of its tetrahedral sites. Likewise, arsenic pentoxide can also dissolve up to an equimolar amount of antimony pentoxide, as antimony substitutes for arsenic only in its octahedral sites. Synthesis Historical Paracelsus Macquer found a crystallizable salt which he ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsenic Trifluoride
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water. Preparation and properties It can be prepared by reacting hydrogen fluoride, HF, with arsenic trioxide Arsenic trioxide, sold under the brand name Trisenox among others, is an inorganic compound and medication. As an industrial chemical, whose major uses include in the manufacture of wood preservatives, pesticides, and glass. As a medication, it ...: :6HF + As2O3 → 2AsF3 + 3H2O It has a pyramidal molecular structure in the gas phase which is also present in the solid. In the gas phase the As-F bond length is 170.6 pm and the F-As-F bond angle 96.2°. Arsenic trifluoride is used as a fluorinating agent for the conversion of non-metal chlorides to fluorides, in this respect it is less reactive than SbF3. Salts containing AsF4− anion can be prepared for example CsAsF4. the potassium salt KAs2F7 prepared from KF an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |