|
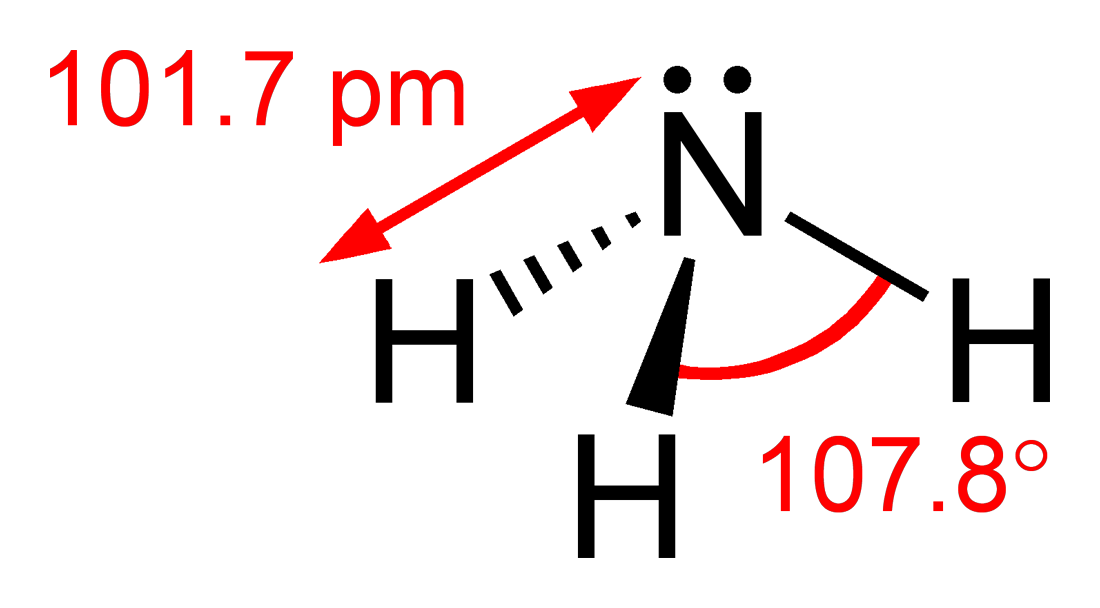
Ammoniac
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both causti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Hydroxide
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests an alkali with chemical formula, composition , it is actually impossible to isolate samples of NH4OH. The ions and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions. Basicity of ammonia in water In aqueous solution, ammonia deprotonation, deprotonates a small fraction of the water to give ammonium and hydroxide according to the following chemical equilibrium, equilibrium: : NH3 + H2O NH4+ + OH−. In a 1 Molar concentration, M ammonia solution, about 0.42% of the ammonia is converted to ammonium, equivalent to pH = 11.63 because [NH4+] = 0.0042 M, [OH−] = 0.0042 M, [NH3] = 0.9958 M, and pH = 14 + log10[OH� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azane
Azanes are acyclic, saturated hydronitrogens, which means that they consist only of hydrogen and nitrogen atoms and all bonds are single bonds. They are therefore pnictogen hydrides. Because cyclic hydronitrogens are excluded by definition, the azanes comprise a homologous series of inorganic compounds with the general chemical formula . Each nitrogen atom has three bonds (either N-H or N-N bonds), and each hydrogen atom is joined to a nitrogen atom (H-N bonds). A series of linked nitrogen atoms is known as the nitrogen skeleton or nitrogen backbone. The number of nitrogen atoms is used to define the size of the azane (e.g. N2-azane). The simplest possible azane (the parent molecule) is ammonia, . There is no limit to the number of nitrogen atoms that can be linked together, the only limitation being that the molecule is acyclic, is saturated, and is a hydronitrogen. Azanes are reactive and have significant biological activity. Azanes can be viewed as a more biologically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties At its standard state, arsine is a colorless, denser-than-air gas that is slightly soluble in water (20% at 20 °C) and in many organic solvents as well. Whereas arsine itself is odorless, owing to its oxidation by air, it is possible to smell a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 230&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods. Historically fertilization came from natural or organic sources: compost, animal manure, human manure, harvested minerals, crop rotations and byproducts of human-nature industries (i.e. fish processing waste, or bloodmeal from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food
Food is any substance consumed by an organism for nutritional support. Food is usually of plant, animal, or fungal origin, and contains essential nutrients, such as carbohydrates, fats, proteins, vitamins, or minerals. The substance is ingested by an organism and assimilated by the organism's cells to provide energy, maintain life, or stimulate growth. Different species of animals have different feeding behaviours that satisfy the needs of their unique metabolisms, often evolved to fill a specific ecological niche within specific geographical contexts. Omnivorous humans are highly adaptable and have adapted to obtain food in many different ecosystems. The majority of the food energy required is supplied by the industrial food industry, which produces food with intensive agriculture and distributes it through complex food processing and food distribution systems. This system of conventional agriculture relies heavily on fossil fuels, which means that the food and agricu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nutrition
Nutrition is the biochemical and physiological process by which an organism uses food to support its life. It provides organisms with nutrients, which can be metabolized to create energy and chemical structures. Failure to obtain sufficient nutrients causes malnutrition. Nutritional science is the study of nutrition, though it typically emphasizes human nutrition. The type of organism determines what nutrients it needs and how it obtains them. Organisms obtain nutrients by consuming organic matter, consuming inorganic matter, absorbing light, or some combination of these. Some can produce nutrients internally by consuming basic elements, while some must consume other organisms to obtain preexisting nutrients. All forms of life require carbon, energy, and water as well as various other molecules. Animals require complex nutrients such as carbohydrates, lipids, and proteins, obtaining them by consuming other organisms. Humans have developed agriculture and cooking to replace for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Waste
Metabolic wastes or excrements are substances left over from metabolic processes (such as cellular respiration) which cannot be used by the organism (they are surplus or toxic), and must therefore be excreted. This includes nitrogen compounds, water, CO2, phosphates, sulphates, etc. Animals treat these compounds as excretes. Plants have chemical "machinery" which transforms some of them (primarily the nitrogen compounds) into useful substances. All the metabolic wastes are excreted in a form of water solutes through the excretory organs (nephridia, Malpighian tubules, kidneys), with the exception of CO2, which is excreted together with the water vapor throughout the lungs. The elimination of these compounds enables the chemical homeostasis of the organism. Nitrogen wastes The nitrogen compounds through which excess nitrogen is eliminated from organisms are called nitrogenous wastes () or nitrogen wastes. They are ammonia, urea, uric acid, and creatinine. All of these substance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pnictogen Hydride
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, and bismuth) covalently bonded to hydrogen. Pnictogen trihydrides The simplest series has the chemical formula XH3 (less commonly H3X), with X representing any of the pnictogens. They take on the pyramidal structure (as opposed to the trigonal planar arrangement of the group 13 hydrides), and therefore are polar. These pnictogen trihydrides are generally increasingly unstable and poisonous with heavier elements. Like the simple hydrogen halides and chalcogenides, the pnictogen hydrides are water- soluble. Unlike other hydrides such as hydrogen sulfide and hydrogen fluoride, which form acidic aqueous solutions, ammonia dissolves in water to make ammonium hydroxide which is basic (by forming a hydroxide ion as opposed to hydronium). Phosphine is also water-sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binary Compounds Of Hydrogen
Binary compounds of hydrogen are binary compound, binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types. Overview Binary hydrogen compounds in group 1 element, group 1 are the ionic hydrides (also called saline hydrides) wherein hydrogen is bound electrostatically. Because hydrogen is located somewhat centrally in an electronegative sense, it is necessary for the counterion to be exceptionally electropositive for the hydride to possibly be accurately described as truly behaving ionic. Therefore, this category of hydrides contains only a few members. Hydrides in group 2 element, group 2 are polymeric covalent hydrides. In these, hydrogen forms bridging covalent bonds, usually possessing mediocre degrees of ionic character, which make them difficult to be accurately described as either ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-3D-balls.png)