|
Aluminium Fluoride
Aluminium fluoride refers to inorganic compounds with the formula AlF3·''x''H2O. They are all colorless solids. Anhydrous AlF3 is used in the production of aluminium metal. Several occur as minerals. Occurrence and production Aside from anhydrous AlF3, several hydrates are known. With the formula AlF3·''x''H2O, these compounds include monohydrate (''x'' = 1), two polymorphs of the trihydrate (''x'' = 3), a hexahydrate (''x'' = 6), and a nonahydrate (''x'' = 9). The majority of aluminium fluoride is produced by treating alumina with hydrogen fluoride at 700 °C: Hexafluorosilicic acid may also be used make aluminum fluoride. :H2SiF6 + Al2O3 + 3 H2O → 2 AlF3 + SiO2 + 4 H2O Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate. For small scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide or aluminium metal with hydrogen fluoride. Aluminium fluoride trihydrate is found in nature as the rare mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Bromide
Aluminium bromide is any chemical compound with the empirical formula AlBrx. Aluminium tribromide is the most common form of aluminium bromide. It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as aluminium tribromide hexahydrate (AlBr3·6H2O). Structure The dimeric form of aluminium tribromide (Al2Br6) predominates in the solid state, in solutions in noncoordinating solvents (e.g. CS2), in the melt, and in the gas phase. Only at high temperatures do these dimers break up into monomers: : Al2Br6 → 2 AlBr3 ΔH°diss = 59 kJ/mol The species aluminium monobromide forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al This reaction is reversed at temperatures higher than 1000 °C. Aluminium monobromide has been crystallographically characterized in the form the tetrameric adduct Al4Br4(NEt3)4 (Et = C2H5). This species is electronical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroaluminate Glass
Fluoride glass is a class of non-oxide optical Glass, glasses composed of Fluoride, fluorides of various metals. They can contain heavy metals such as zirconium, or be combined with lighter elements like Aluminium, aluminum and beryllium. These heavier elements cause the glass to have a transparency range extended into the infrared wavelength. Thus, the goal for heavy metal fluoride glasses (HMFG) is to create ultra-low loss optical fiber communication systems for commercial and defense applications as well as bulk components that can be used in invasive medical treatment. However, the heavier elements also cause the glass to have a low viscosity and make them vulnerable to crystallization during the glass transition or processing. This makes the glass more fragile and has poor resistance to moisture and environmental attacks. Fluoride glasses' best attribute is that they lack the absorption band associated with the hydroxyl (OH) group (3.2–3.6 micrometers) which is presen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium Fluoride
Zirconium(IV) fluoride ( Zr F4) is an inorganic chemical compound. It is a component of ZBLAN fluoride glass. It is insoluble in water. It is the main component of fluorozirconate glasses. Three crystalline phases of ZrF4 have been reported, α (monoclinic), β (tetragonal, Pearson symbol tP40, space group P42/m, No 84) and γ (unknown structure). β and γ phases are unstable and irreversibly transform into the α phase at 400 °C. Zirconium fluoride is used as a zirconium source in oxygen-sensitive applications, e.g. metal production. Zirconium fluoride can be purified by distillation or sublimation. Conditions/substances to avoid are: moisture, active metals, acids and oxidizing agents. Zirconium fluoride in a mixture with other fluorides is a coolant for molten salt reactors. In the mixture with sodium fluoride it is a candidate coolant for the Advanced High-Temperature Reactor. Together with uranium salt, zirconium fluoride can be a component of fuel-coolant in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
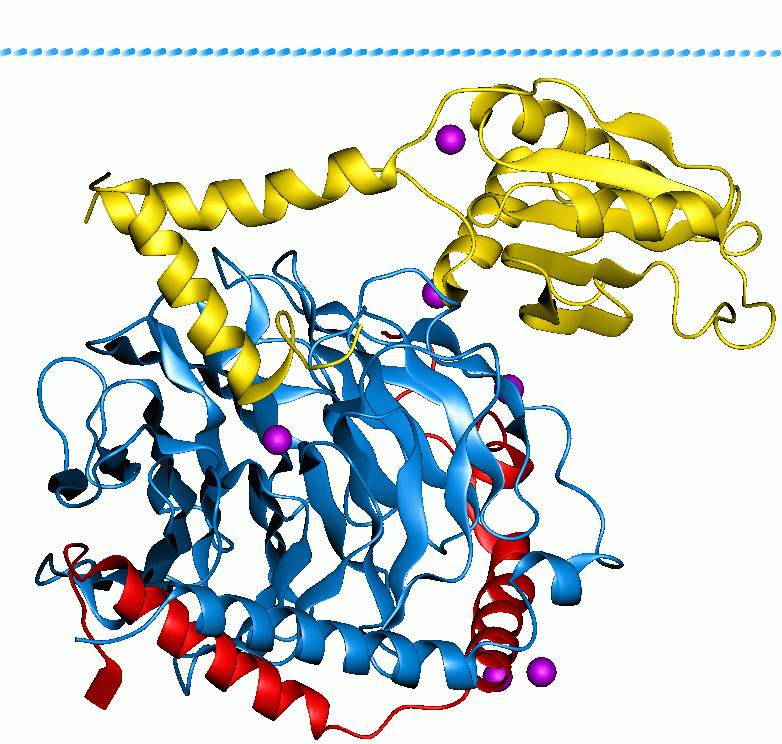
GTPase-activating Protein
GTPase-activating proteins or GTPase-accelerating proteins (GAPs) are a family of regulatory proteins whose members can bind to activated G proteins and stimulate their GTPase activity, with the result of terminating the signaling event. GAPs are also known as RGS protein, or RGS proteins,Kimple, A.J. "Structural Determinants of G-protein α Subunit Selectivity by Regulator of G-protein Signaling 2 (RGS2)". ''The Journal of Biological Chemistry''. 284 (2009): 19402-19411. and these proteins are crucial in controlling the activity of G proteins. Regulation of G proteins is important because these proteins are involved in a variety of important cellular processes. The large G proteins, for example, are involved in transduction of signaling from the G protein-coupled receptor for a variety of signaling processes like hormonal signaling, and small G proteins are involved in processes like cellular trafficking and cell cycling. GAP's role in this function is to turn the G protein's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases. There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of '' alpha'' (α), ''beta'' (β) and ''gamma'' (γ) subunits. In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex . Heterotrimeric G proteins located within the cell are activ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanosine Triphosphate
Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon. It also has the role of a source of energy or an activator of substrates in metabolic reactions, like that of ATP, but more specific. It is used as a source of energy for protein synthesis and gluconeogenesis. GTP is essential to signal transduction, in particular with G-proteins, in second-messenger mechanisms where it is converted to guanosine diphosphate (GDP) through the action of GTPases. Uses Energy transfer GTP is involved in energy transfer within the cell. For instance, a GTP molecule is generated by one of the enz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar (ribose), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryolite
Cryolite ( Na3 Al F6, sodium hexafluoroaluminate) is an uncommon mineral identified with the once-large deposit at Ivittuut on the west coast of Greenland, mined commercially until 1987. History Cryolite was first described in 1798 by Danish veterinarian and physician Peder Christian Abildgaard (1740–1801); it was obtained from a deposit of it in Ivigtut (old spelling) and nearby Arsuk Fjord, Southwest Greenland. The name is derived from the Greek language words ''κρύος'' (cryos) = frost, and ''λίθος'' (lithos) = stone. The Pennsylvania Salt Manufacturing Company used large amounts of cryolite to make caustic soda and fluorine compounds, including hydrofluoric acid at its Natrona, Pennsylvania works, and at its integrated chemical plant in Cornwells Heights, Pennsylvania, during the 19th and 20th centuries. It was historically used as an ore of aluminium and later in the electrolytic processing of the aluminium-rich oxide ore bauxite (itself a combination of aluminiu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |