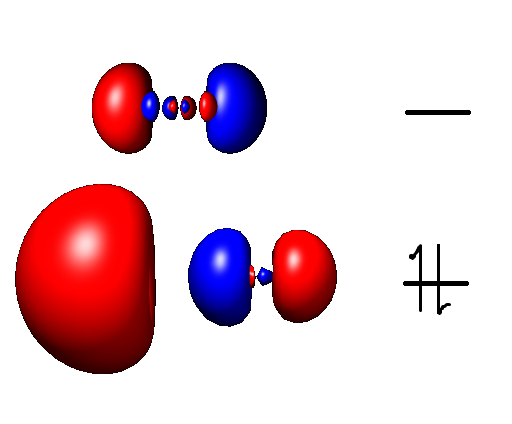|
Aluminium(I) Compounds
In chemistry, aluminium(I) refers to monovalent aluminium (+1 oxidation state) in both ionic and covalent bonds. Along with aluminium(II), it is an extremely unstable form of aluminium. While late Group 13 elements such as thallium and indium prefer the +1 oxidation state, aluminium(I) is rare. Aluminium does not experience the inert pair effect, a phenomenon where valence s electrons are poorly shielded from nuclear charge due to the presence of filled d and f orbitals. As such, aluminium (III) (Al^3+) is the much more common oxidation state for aluminium. Aluminium(I) compounds are both prone to disproportionation and difficult to prepare. At standard conditions, they readily oxidize to the aluminium(III) form. Characteristics Al(I) appears to be red, as solutions of AlBr and AlCl in organic solvents are both red. The presence of this color implies a relatively small HOMO/LUMO gap that is accessible by green light. The geometry of compounds can be determined by analysis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monovalent Ion
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1. Chlorine, as it has a valence of one, can be substituted for hydrogen. Phosphorus has a valence of 5 in phosphorus pentachloride, . Valence diagrams of a compound represent the connectivity of the elements, with lines drawn between two elements, sometimes called bonds, representing a saturated valency for each element. The two tables below show some examples of different compounds, their valence diagrams, and the valences for each element of the compound. Modern definitions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monoiodide
Aluminium monoiodide is an aluminium(I) compound with the chemical formula AlI. It is unstable at room temperature due to dismutation: :6AlI -> + 4Al It forms a cyclic adduct Al4I4(NEt3)4 with triethylamine. See also *Aluminium monofluoride * Aluminium monochloride *Aluminium monobromide Aluminium monobromide is a chemical compound with the empirical formula AlBr. It forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al This reaction i ... References Aluminium(I) compounds Iodides Metal halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P4 And Nacnacal(I)
P4 may refer to: Computing * Intel Pentium 4, a 7th-generation processor design ** The P4 power connector introduced to power it and later CPUs in the ATX12V 1.0 standard * The i486DX (P4) model of the Intel 80486, a high-performance microprocessor * P4 (programming language), for describing packet forwarding * P4, the Perforce software command line client Media * P4 Radio Hele Norge (PFI), a Norwegian radio company ** Kanal 24 (Kanal 4), which acquired the Norwegian P4 channel from PFI * Sveriges Radio P4, a Swedish radio channel * ''Persona 4'', a 2008 video game * '' Periphery IV: Hail Stan'', 2019 album by American progressive metal band Periphery Military * Skaraborg Regiment (armoured), a Swedish army unit whose designation is P 4 * Peugeot P4, a vehicle used by the French Military Science * P4 laboratory, a biosafety level 4 facility * Tetraphosphorus (P4), an allotrope of phosphorus * Group p4, the plane symmetry group Wallpaper group p4 * Progesterone (Pregn-4-ene-3, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene Versus Aluminum (I)
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene, the parent hydride from which all other carbene compounds are formally derived. Carbenes are classified as either singlets or triplets, depending upon their electronic structure. Most carbenes are very short lived, although persistent carbenes are known. One well-studied carbene is dichlorocarbene , which can be generated '' in situ'' from chloroform and a strong base. Structures and bonding The two classes of carbenes are singlet and triplet carbenes. Singlet carbenes are spin-paired. In the language of valence bond theory, the molecule adopts an sp2 hybrid structure. Triplet carbenes have two unpaired electrons. Most carbenes have a nonlinear triplet ground state, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenes
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene, the parent hydride from which all other carbene compounds are formally derived. Carbenes are classified as either singlets or triplets, depending upon their electronic structure. Most carbenes are very short lived, although persistent carbenes are known. One well-studied carbene is dichlorocarbene , which can be generated ''in situ'' from chloroform and a strong base. Structures and bonding The two classes of carbenes are singlet and triplet carbenes. Singlet carbenes are spin-paired. In the language of valence bond theory, the molecule adopts an sp2 hybrid structure. Triplet carbenes have two unpaired electrons. Most carbenes have a nonlinear triplet ground stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthesis Of Nacnacal(i) Generic
Synthesis or synthesize may refer to: Science Chemistry and biochemistry *Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors ** Organic synthesis, the chemical synthesis of organic compounds ***Total synthesis, the complete organic synthesis of complex organic compounds, usually without the aid of biological processes ***Convergent synthesis or linear synthesis, a strategy to improve the efficiency of multi-step chemical syntheses **Dehydration synthesis, a chemical synthesis resulting in the loss of a water molecule * Biosynthesis, the creation of an organic compound in a living organism, usually aided by enzymes **Photosynthesis, a biochemical reaction using a carbon molecule to produce an organic molecule, using sunlight as a catalyst **Chemosynthesis, the synthesis of biological compounds into organic waste, using methane or an oxidized molecule as a catalyst **Amino acid synthesis, the synthesis of an amino ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem Structure Of Hnacnac
Chem may refer to: * Chemistry practical waali mam * Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" * Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel *CHEM-DT CHEM-DT is the TVA owned-and-operated television station in Trois-Rivières, Quebec, Canada. It broadcasts a high-definition digital signal on VHF channel 8 from a transmitter on Rue Principale in Notre-Dame-du-Mont-Carmel. Owned by the Grou ..., a Canadian television channel See also * Chemo (other) * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Abundance Of Elements In Earth's Crust
The abundance of elements in Earth's crust is shown in tabulated form with the estimated crustal abundance for each chemical element shown as mg/kg, or parts per million (ppm) by mass (10,000 ppm = 1%). Estimates of elemental abundance are difficult because (a) the composition of the upper and lower crust are quite different, and (b) the composition of the continental crust can vary drastically by locality. David KringComposition of Earth's continental crust as inferred from the compositions of impact melt sheets Lunar and Planetary Science XXVIII List of abundance by element See also * Abundances of the elements (data page) * Atmospheric chemistry * Clarke number — lists of historical data and terminology * List of chemical elements This is a list of the 118 chemical elements which have been identified as of 2022. A chemical element, often simply called an element, is a type of atom which has the same number of protons in its atomic nucleus (i.e., the same atomic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Fluoride
Aluminium fluoride refers to inorganic compounds with the formula AlF3·''x''H2O. They are all colorless solids. Anhydrous AlF3 is used in the production of aluminium metal. Several occur as minerals. Occurrence and production Aside from anhydrous AlF3, several hydrates are known. With the formula AlF3·''x''H2O, these compounds include monohydrate (''x'' = 1), two polymorphs of the trihydrate (''x'' = 3), a hexahydrate (''x'' = 6), and a nonahydrate (''x'' = 9). The majority of aluminium fluoride is produced by treating alumina with hydrogen fluoride at 700 °C: Hexafluorosilicic acid may also be used make aluminum fluoride. :H2SiF6 + Al2O3 + 3 H2O → 2 AlF3 + SiO2 + 4 H2O Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate. For small scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide or aluminium metal with hydrogen fluoride. Aluminium fluoride trihydrate is found in nature as the rare mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comproportionation
Comproportionation or synproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionation.Shriver, D. F.; Atkins, P. W.; Overton, T. L.; Rourke, J. P.; Weller, M. T.; Armstrong, F. A. “Inorganic Chemistry” W. H. Freeman, New York, 2006. . Frost diagrams The tendency of two species to disproportionate or comproportionate can be determined by examining the Frost diagram of the oxidation states; if a species' value of Δ''G''/''F'' is lower than the line joining the two oxidation numbers on either side of it, then it is more stable and if in a solution, these two species will undergo comproportionation. A Frost Diagram is another way of displaying the reduction potentials for the various oxidation states of a given element, X. It shows nE against the oxidation number N: here, E is the reduction potential for the X(N)/X(0) c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with discoveri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |