|
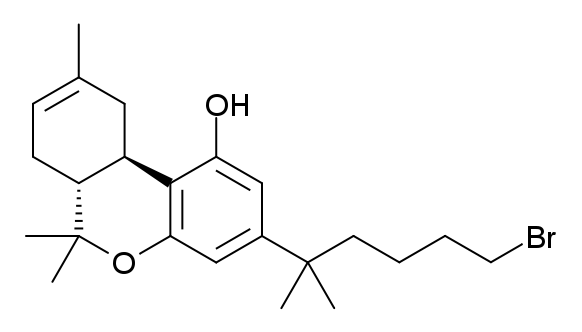
AM-2232
AM-2232 (1-(4-cyanobutyl)-3-(naphthalen-1-oyl)indole) is a drug that acts as a potent but unselective agonist for the cannabinoid receptors, with a ''K''i of 0.28 nM at CB1 and 1.48 nM at CB2. In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as AM-2232 are Schedule I Controlled Substances. See also * AM-694 * AM-1235 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * O-774 * O-1057 * O-1812 * THJ-2201 THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is a structural analog of AM-2201 in which the central indole ring has been replac ... References Naphthoylindoles Nitriles AM cannabinoids Designer drugs {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally ... References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM Cannabinoids
Alexandros Makriyannis is a professor in the Department of Medicinal Chemistry at Northeastern University, where his research group has synthesized many new compounds with cannabinoid activity. Some of those are: See also * List of CP cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of miscellaneous designer cannabinoids Since the first synthetic cannabinoids were discovered in recreational drug products in 2008, new synthetic cannabinoids with no precedent in the scientific literature continue to be identified. These synthetic cannabinoids appear to be rationally ... References {{Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-2233
AM-2233 is a drug that acts as a highly potent full agonist for the cannabinoid receptors, with a ''K''i of 1.8 nM at CB1 and 2.2 nM at CB2 as the active (''R'') enantiomer. It was developed as a selective radioligand for the cannabinoid receptors and has been used as its 131I derivative for mapping the distribution of the CB1 receptor in the brain. AM-2233 was found to fully substitute for THC in rats, with a potency lower than that of JWH-018 but higher than WIN 55,212-2. It is notable for inducing tinnitus, though the reasons for this are unclear and may provide valuable insight into tinnitus research. Legal Status As of October 2015 AM-2233 is a controlled substance in China. See also * AM-679 * AM-694 * AM-1220 * AM-1221 * AM-1235 * AM-1241 * AM-2232 * Cannabipiperidiethanone * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * List of HU cannabinoids * List of designer drugs Designer drugs are structural or functional analogues of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
THJ-2201
THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is a structural analog of AM-2201 in which the central indole ring has been replaced by indazole. Pharmacology THJ-2201 acts as a full agonist with a binding affinity of 1.34nM at CB1 and 1.32nM at CB2 cannabinoid receptors. Side effects THJ-2201 has been linked to at least one hospitalization and death due to its use. Legal status Because of the hazards associated with recreational use of this compound, it is classified as a Schedule I controlled substance in the United States. It is also an Anlage II controlled drug in Germany. See also * AM-694 * AM-1235 * AM-2232 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * NM-2201 * THJ-018 THJ-018 (SGT-17) is a synthetic cannabinoid that is the indazole analogue of JWH-018 and has been sold online as a designer drug. Pharmaco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-1812
O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a ''K''i of 3.4 nM at CB1 and 3870 nM at CB2. Unlike most related compounds, O-1812 is metabolically stable against rapid breakdown by enzymes, and produces a cannabinoid-like discriminative effect in rats, which is similar but not identical to that produced by cannabinoid drugs of other chemical classes. See also * AM-1235 * AM-2232 * AM-2389 * Methanandamide * O-774 * O-1057 O-1057 is an analgesic cannabinoid derivative created by Organix Inc., Newburyport, Massachusetts, for use in scientific research. Unlike most cannabinoids discovered to date, it is water-soluble, which gives it considerable advantages over many ... References Cannabinoids Nitriles Fatty acid amides {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-1057
O-1057 is an analgesic cannabinoid derivative created by Organix Inc., Newburyport, Massachusetts, for use in scientific research. Unlike most cannabinoids discovered to date, it is water-soluble, which gives it considerable advantages over many related cannabinoids. It has moderate affinity for both CB1 and CB2 receptors, with ''K''i values of 8.36 nM at CB1 and 7.95 nM at CB2 See also * AM-2232 * O-774 * O-1812 * O-2694 * SP-111 THC morpholinylbutyrate (SP-111, Δ9-THC-O- -(morpholin-4-yl)butyrate'') is a synthetic derivative of tetrahydrocannabinol, developed in the 1970s. It is a prodrug which is converted into THC inside the body, and was one of the first derivatives ... References {{cannabinoid-stub Cannabinoids Benzochromenes Carboxylate esters 4-Morpholinyl compunds Nitriles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-774
O-774 is a classical cannabinoid derivative which acts as a potent agonist for the cannabinoid receptors, with a ''K''i of 0.6 nM at CB1, and very potent cannabinoid effects in animal studies. See also * AM-2232 * O-1057 * O-1812 O-1812 is an eicosanoid derivative related to anandamide that acts as a potent and highly selective agonist for the cannabinoid receptor CB1, with a ''K''i of 3.4 nM at CB1 and 3870 nM at CB2. Unlike most related compounds, O-1812 is ... References Benzochromenes Cannabinoids {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
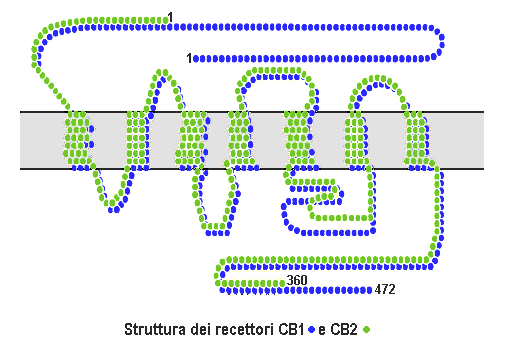
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FUBIMINA
FUBIMINA (also known as BIM-2201, BZ-2201 and FTHJ) is a synthetic cannabinoid that is the benzimidazole analog of AM-2201 and has been used as an active ingredient in synthetic cannabis products. It was first identified in Japan in 2013, alongside MEPIRAPIM. FUBIMINA acts as a reasonably potent agonist for the CB2 receptor ( ''K''i = 23.45 nM), with 12x selectivity over CB1 (''K''i = 296.1 nM), and does not fully substitute for Δ9-THC in rat discrimination studies. Related benzimidazole derivatives have been reported to be highly selective agonists for the CB2 receptor. See also * AM-694 * AM-1235 * AM-2232 * AM-2233 * BIM-018 * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * THJ-2201 THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is a structural analog of AM-2201 in which the central indole ring has been r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-1235
AM-1235 (1-(5-fluoropentyl)-3-(naphthalen-1-oyl)-6-nitroindole) is a drug that acts as a potent and reasonably selective agonist for the cannabinoid receptor CB1. Pharmacology Pharmacodynamics AM-1235 is a cannabinoid receptor agonist with Ki of 1.5 nM at CB1 compared to 20.4 nM at CB2. While the 6-nitro substitution on the indole ring reduces affinity for both CB1 and CB2 relative to the unsubstituted parent compound AM-2201, CB2 affinity is reduced much more, resulting in a CB1 selectivity of around 13 times. This is in contrast to other related compounds such as AM-1221 where a 6-nitro substitution instead confers significant selectivity for CB2. Pharmacokinetics AM-1235 metabolism differs only slightly from that of JWH-018. AM-1235 ''N''-dealkylation produces fluoropentane instead of pentane (or plain alkanes in general). It has been speculated that the fluoropentane might function as an alkylating agent or is further metabolized into toxic fluoroacetic acid. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-694
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors. Pharmacology AM-694 is an agonist for cannabinoid receptors. It has a ''K''i of 0.08 nM at CB1 and 18 times selectivity over CB2 with a ''K''i of 1.44 nM. It is unclear what is responsible for this unusually high CB1 binding affinity, but it makes the 18F radiolabelled derivative of AM-694 useful for mapping the distribution of CB1 receptors in the body. Metabolism Pathways of metabolism include hydrolytic defluorination, carboxylation, and monohydroxylation of the ''N''-alkyl chain. See also * AM-679 * AM-1235 * AM-2201 * AM-2232 * AM-2233 * FUBIMINA * JWH-018 * List of AM cannabinoids * List of JWH cannabinoids * THJ-2201 THJ-2201 is an indazole-based synthetic cannabinoid that presumably acts as a potent agonist of the CB1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 2 (macrophage)
The cannabinoid receptor type 2, abbreviated as CB2, is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor type 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor type 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |