|
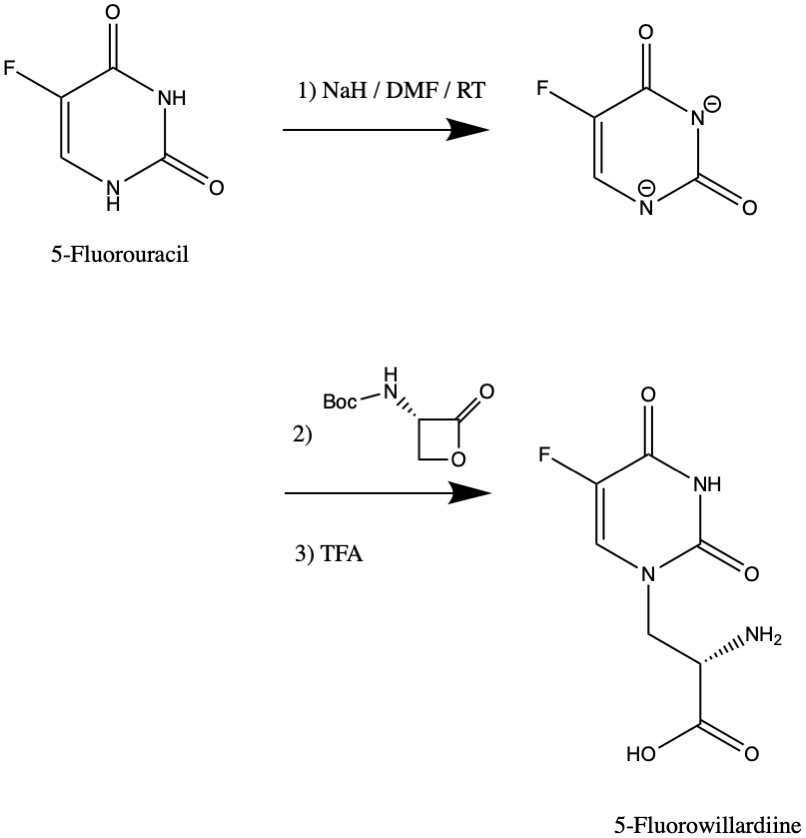
5-Fluorowillardiine
5-Fluorowillardiine is a selective agonist for the AMPA receptor, with only limited effects at the kainate receptor. It is an excitotoxic neurotoxin when used ''in vivo'' and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors ''in vitro''. It is structurally similar to the compound willardiine, which is also an agonist for the AMPA and kainate receptors. Willardiine occurs naturally in Mariosousa willardiana and Acacia sensu lato. The name is unusual as it has two successive i's. This is not a typo. Toxicity (S)-5-Fluorowillardiine activity has been studied ''in vitro'' in a variety of neural tissues. In mouse embryo hippocampal neurons, it was found to desensitize AMPA/kainate receptors with an EC50 of 1.5 μM –— 7 times more potent than racemic AMPA (EC50 of 11 μM). In another study, (S)-5-Fluorowillardiine showed biphasic dose-dependent neurotoxicity in cultural rodent cortical neurons, with EC50 vales of 0.70 and 170 � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-fluorowillardiine
5-Fluorowillardiine is a selective agonist for the AMPA receptor, with only limited effects at the kainate receptor. It is an excitotoxic neurotoxin when used ''in vivo'' and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors ''in vitro''. It is structurally similar to the compound willardiine, which is also an agonist for the AMPA and kainate receptors. Willardiine occurs naturally in Mariosousa willardiana and Acacia sensu lato. The name is unusual as it has two successive i's. This is not a typo. Toxicity (S)-5-Fluorowillardiine activity has been studied ''in vitro'' in a variety of neural tissues. In mouse embryo hippocampal neurons, it was found to desensitize AMPA/kainate receptors with an EC50 of 1.5 μM –— 7 times more potent than racemic AMPA (EC50 of 11 μM). In another study, (S)-5-Fluorowillardiine showed biphasic dose-dependent neurotoxicity in cultural rodent cortical neurons, with EC50 vales of 0.70 and 170 � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AMPA Receptor
The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (also known as AMPA receptor, AMPAR, or quisqualate receptor) is an ionotropic receptor, ionotropic transmembrane receptor for glutamate (iGluR) that mediates fast synapse, synaptic transmission in the central nervous system (CNS). It has been traditionally classified as a non-NMDA_receptor, NMDA-type receptor, along with the kainate receptor. Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualic acid, quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The ''GRIA2''-encoded AMPA receptor ligand binding core (GluA2 LBD) was the first glutamate receptor ion channel domain to be protein crystal, crystallized. Structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Organofluorides
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AMPA Receptor Agonists
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, better known as AMPA, is a compound that is a specific agonist for the AMPA receptor, where it mimics the effects of the neurotransmitter glutamate. There are several types of glutamatergic ion channels in the central nervous system including AMPA, kainic acid and ''N''-methyl-D-aspartic acid (NMDA) channels. In the synapse, these receptors serve very different purposes. AMPA can be used experimentally to distinguish the activity of one receptor from the other in order to understand their differing functions. AMPA generates fast excitatory postsynaptic potentials (EPSP). AMPA activates AMPA receptors that are non-selective cationic channels allowing the passage of Na+ and K+ and therefore have an equilibrium potential near 0 mV. AMPA was first synthesized, along with several other ibotenic acid Ibotenic acid or (''S'')-2-amino-2-(3-hydroxyisoxazol-5-yl)acetic acid, also referred to as ibotenate, is a chemical compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strecker Amino Acid Synthesis
The Strecker amino acid synthesis, also known simply as the Strecker synthesis, is a method for the synthesis of amino acids by the reaction of an aldehyde with ammonia in the presence of potassium cyanide. The condensation reaction yields an α-aminonitrile, which is subsequently hydrolyzed to give the desired amino acid. The method is used commercially for the production of racemic methionine from methional. While usage of ammonium salts gives unsubstituted amino acids, primary and secondary amines also give substituted amino acids. Likewise, the usage of ketones, instead of aldehydes, gives α,α-disubstituted amino acids. Reaction mechanism In the first part of the reaction, the carbonyl oxygen of an aldehyde is protonated, followed by a nucleophilic attack of ammonia to the carbonyl carbon. After subsequent proton exchange, water is cleaved from the iminium ion intermediate. A cyanide ion then attacks the iminium carbon yielding an aminonitrile. In the second part of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SN2 Reaction
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed in a concerted way, i.e., in one step. The name SN2 refers to the Hughes-Ingold symbol of the mechanism: "SN" indicates that the reaction is a nucleophilic substitution, and "2" that it proceeds via a bi-molecular mechanism, which means both the reacting species are involved in the rate-determining step. The other major type of nucleophilic substitution is the SN1, but many other more specialized mechanisms describe substitution reactions. The SN2 reaction can be considered as an analogue of the associative substitution in the field of inorganic chemistry. Reaction mechanism The reaction most often occurs at an aliphatic sp3 carbon center with an electronegative, stable leaving group attached to it (often denoted X), which is frequently a halide atom. The breaking of the C–X bond and the formation of the new bond (often deno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-Fluorouracil
Fluorouracil (5-FU), sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pancreatic cancer, breast cancer, and cervical cancer. As a cream it is used for actinic keratosis, basal cell carcinoma, and skin warts. Side effects of use by injection are common. They may include inflammation of the mouth, loss of appetite, low blood cell counts, hair loss, and inflammation of the skin. When used as a cream, irritation at the site of application usually occurs. Use of either form in pregnancy may harm the baby. Fluorouracil is in the antimetabolite and pyrimidine analog families of medications. How it works is not entirely clear, but it is believed to involve blocking the action of thymidylate synthase and thus stopping the production of DNA. Fluorouracil was patented in 1956 and came into medical use in 1962. It is on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomers
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uracil
Uracil () (symbol U or Ura) is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine (T). Uracil is a demethylated form of thymine. Uracil is a common and naturally occurring pyrimidine derivative. The name "uracil" was coined in 1885 by the German chemist Robert Behrend, who was attempting to synthesize derivatives of uric acid. Originally discovered in 1900 by Alberto Ascoli, it was isolated by hydrolysis of yeast nuclein; it was also found in bovine thymus and spleen, herring sperm, and wheat germ. It is a planar, unsaturated compound that has the ability to absorb light. Based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, it is believed that uracil, xanthine, and related molecules can also be formed extraterrestrially. Data from the Cassini mission, orbiting in the Saturn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionotropic Glutamate Receptors
Ionotropic glutamate receptors (iGluRs) are ligand-gated ion channels that are activated by the neurotransmitter glutamate. They mediate the majority of excitatory synaptic transmission throughout the central nervous system and are key players in synaptic plasticity, which is important for learning and memory. iGluRs have been divided into four subtypes on the basis of their ligand binding properties ( pharmacology) and sequence similarity: AMPA receptors, kainate receptors, NMDA receptors and delta receptors (see below). AMPA receptors are the main charge carriers during basal transmission, permitting influx of sodium ions to depolarise the postsynaptic membrane. NMDA receptors are blocked by magnesium ions and therefore only permit ion flux following prior depolarisation. This enables them to act as coincidence detectors for synaptic plasticity. Calcium influx through NMDA receptors leads to persistent modifications in the strength of synaptic transmission. iGluRs are tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |