Isomers on:
[Wikipedia]
[Google]
[Amazon]
In
"isomer"
online dictionary entry. Accessed on 2020-08-26 which was borrowed through
"isomeric"
online dictionary entry. Accessed on 2020-08-26 from
C3H8O :
 The first two isomers shown of
The first two isomers shown of C3H8O are -OH comprising the H3C-CH2-CH2OH and H3C-CH(OH)-CH3 .
The third isomer of C3H8O is the H3C-O-CH2-CH3 .
The alcohol "3-propanol" is not another isomer, since the difference between it and 1-propanol is not real; it is only the result of an arbitrary choice in the direction of numbering the carbons along the chain. For the same reason, "ethoxymethane" is the same molecule as methoxyethane, not another isomer.
1-Propanol and 2-propanol are examples of positional isomers, which differ by the position at which certain features, such as
C3H4 :
In two of the isomers, the three carbon atoms are connected in an open chain, but in one of them (
H-X-Y=Z <=> X=Y-Z-H .
Important examples are
C8H10 with a
A to another energy minimum B will therefore require going through configurations that have higher energy than A and B . That is, a conformation isomer is separated from any other isomer by an  A classic example of conformational isomerism is
A classic example of conformational isomerism is C-C-C angles are close to 110 degrees. Conformations of the cyclohexane molecule with all six carbon atoms on the same plane have a higher energy, because some or all the C-C-C angles must be far from that value (120 degrees for a regular hexagon). Thus the conformations which are local energy minima have the ring twisted in space, according to one of two patterns known as NHFCl or H2O2 , because the two conformations with minimum energy interconvert in a few
Methods of conformational analysis
. Chapter 2 in ''Conformation of Carbohydrates''. 409 pages. (However, one should be aware that the terms "conformation" and "configuration" are largely synonymous outside of chemistry, and their distinction may be controversial even among chemists.Anatoly M Belostotskii (2015):
Conformer and conformation
, chapter 2 of ''Conformational Concept For Synthetic Chemist's Use: Principles And in Lab Exploitation''. 580 pages. .) Interactions with other molecules of the same or different compounds (for example, through
CHFClBr ). The two enantiomers can be distinguished, for example, by whether the path F->Cl->Br turns clockwise or counterclockwise as seen from the hydrogen atom. In order to change one conformation to the other, at some point those four atoms would have to lie on the same plane – which would require severely straining or breaking their bonds to the carbon atom. The corresponding energy barrier between the two conformations is so high that there is practically no conversion between them at room temperature, and they can be regarded as different configurations.
The compound CH2ClF , in contrast, is not chiral: the mirror image of its molecule is also obtained by a half-turn about a suitable axis.
Another example of a chiral compound is 2,3-pentadiene H3C-CH=C=CH-CH3 a hydrocarbon that contains two overlapping double bonds. The double bonds are such that the three middle carbons are in a straight line, while the first three and last three lie on perpendicular planes. The molecule and its mirror image are not superimposable, even though the molecule has an axis of symmetry. The two enantiomers can be distinguished, for example, by the
C2H2Cl2 , specifically the structural isomer Cl-HC=CH-Cl that has one chlorine bonded to each carbon. It has two conformational isomers, with the two chlorines on the same side or on opposite sides of the double bond's plane. They are traditionally called ''cis'' (from Latin meaning "on this side of") and ''trans'' ("on the other side of"), respectively; or ''Z'' and ''E'' in the (CHOH)6 (a six-fold alcohol of cyclohexane), the six-carbon cyclic backbone largely prevents the hydroxyl -OH and the hydrogen -H on each carbon from switching places. Therefore, one has different configurational isomers depending on whether each hydroxyl is on "this side" or "the other side" of the ring's mean plane. Discounting isomers that are equivalent under rotations, there are nine isomers that differ by this criterion, and behave as different stable substances (two of them being enantiomers of each other). The most common one in nature (''myo''-inositol) has the hydroxyls on carbons 1, 2, 3 and 5 on the same side of that plane, and can therefore be called ''cis''-1,2,3,5-''trans''-4,6-cyclohexanehexol. And each of these ''cis''-''trans'' isomers can possibly have stable "chair" or "boat" conformations (although the barriers between these are significantly lower than those between different ''cis''-''trans'' isomers).
''Cis'' and ''trans'' isomers also occur in inorganic MX2Y2 complexes and MX4Y2 complexes.
For more complex organic molecules, the ''cis'' and ''trans'' labels are ambiguous. The IUPAC recommends a more precise labeling scheme, based on the CIP priorities for the bonds at each carbon atom.
PF4Cl , the bonds from the PF3Cl2 , three isomers are possible, with zero, one, or two chlorines in the axial positions.
As another example, a complex with a formula like MX3Y3 , where the central atom M forms six bonds with X bonds (and thus also the three Y bonds) are directed at the three corners of one face of the octahedron (''fac'' isomer), or lie on the same equatorial or "meridian" plane of it (''mer'' isomer).
H3C-CH3 , all the bond angles and length are narrowly constrained, except that the two C-C axis. Thus, even if those angles and distances are assumed fixed, there are infinitely many conformations for the ethane molecule, that differ by the relative angle φ of rotation between the two groups. The feeble repulsion between the hydrogen atoms in the two methyl groups causes the energy to minimized for three specific values of φ, 120° apart. In those configurations, the six planes H-C-C or C-C-H are 60° apart. Discounting rotations of the whole molecule, that configuration is a single isomer – the so-called ''staggered'' conformation.
Rotation between the two halves of the molecule ClH2C-CH2Cl also has three local energy minima, but they have different energies due to differences between the H-H , Cl-Cl , and H-Cl interactions. There are therefore three rotamers: a ''trans'' isomer where the two chlorines are on the same plane as the two carbons, but with oppositely directed bonds; and two ''gauche'' isomers, mirror images of each other, where the two -CH2Cl groups are rotated about 109° from that position. The computed energy difference between ''trans'' and ''gauche'' is ~1.5 kcal/mol, the barrier for the ~109° rotation from ''trans'' to ''gauche'' is ~5 kcal/mol, and that of the ~142° rotation from one ''gauche'' to its enantiomer is ~8 kcal/mol.Kenneth B. Wiberg and Mark A. Murcko (1987): "Rotational barriers. 1. 1,2-Dihaloethanes". ''Journal of Physical Chemistry'', volume 91, issue 13, pages 3616–3620. The situation for
^1 H ) by ^2 H , or D ) on an HD2C-CH3 ) or one on each carbon (1,2-dideuteroethane, DH2C-CDH2 ); as if the substituent was CH2ClF ). While the original molecule is not chiral and has a single isomer, the substitution creates a pair of chiral enantiomers of CHDClF , which could be distinguished (at least in theory) by their optical activity.
When two isomers would be identical if all isotopes of each element were replaced by a single isotope, they are described as D were replaced by H , the two dideuteroethanes would both become ethane and the two deuterochlorofluoromethanes would both become CH2ClF .
The concept of isotopomers is different from isotopologs or isotopic homologs, which differ in their isotopic composition. For example, C2H5D and C2H4D2 are isotopologues and not isotopomers, and are therefore not isomers of each other.
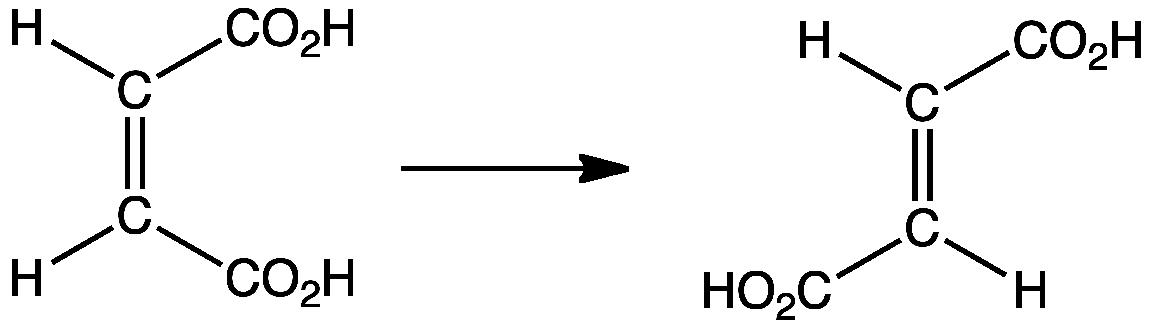
 ;Synthesis of fumaric acid
Industrial synthesis of fumaric acid proceeds via the cis-trans isomerization of maleic acid:
:
;Synthesis of fumaric acid
Industrial synthesis of fumaric acid proceeds via the cis-trans isomerization of maleic acid:
:
AgCNO was identical to O- N+ ≡C- and O=C=N- , respectively.)
Additional examples were found in succeeding years, such as Wöhler's 1828 discovery that CH4N2O ) as the chemically distinct (H2N-)2C=O and
Om sammansättningen af vinsyra och drufsyra (John's säure aus den Voghesen), om blyoxidens atomvigt, samt allmänna anmärkningar om sådana kroppar som hafva lika sammansättning, men skiljaktiga egenskaper
("On the composition of tartaric acid and racemic acid (John's acid of the Vosges), on the molecular weight of lead oxide, together with general observations on those bodies that have the same composition but distinct properties"). ''Kongliga Svenska Vetenskaps Academiens Handling'' (''Transactions of the Royal Swedish Science Academy''), volume 49, pages 49–80J. J. Berzelius (1831):
Über die Zusammensetzung der Weinsäure und Traubensäure (John's säure aus den Voghesen), über das Atomengewicht des Bleioxyds, nebst allgemeinen Bemerkungen über solche Körper, die gleiche Zusammensetzung, aber ungleiche Eigenschaften besitzen
. ''Annalen der Physik und Chemie'', volume 19, pages 305–335J. J. Berzelius (1831):
Composition de l'acide tartarique et de l'acide racémique (traubensäure); poids atomique de l'oxide de plomb, et remarques générals sur les corps qui ont la même composition, et possèdent des proprietés différentes
. ''Annales de Chimie et de Physique'', volume 46, pages 113–147. In 1848,
"Sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire"
("On the relations that can exist between crystalline form, chemical composition, and the sense of rotary polarization"), ''Annales de Chimie et de Physique'', 3rd series, volume 24, issue 6, pages 442–459.
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, isomers are molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
s or polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may not ...
s with identical molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
e – that is, same number of atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Isomers do not necessarily share similar chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wi ...
or physical properties
A physical property is any property that is measurable, whose value describes a state of a physical system. The changes in the physical properties of a system can be used to describe its changes between momentary states. Physical properties are o ...
. Two main forms of isomerism are structural
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such ...
or constitutional isomerism, in which ''bond
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemica ...
s'' between the atoms differ; and stereoisomerism
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ.
Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest.
The English word "isomer" () is a back-formation
In etymology, back-formation is the process or result of creating a new word via inflection, typically by removing or substituting actual or supposed affixes from a lexical item, in a way that expands the number of lexemes associated with the c ...
from "isomeric",Merriam-Webster"isomer"
online dictionary entry. Accessed on 2020-08-26 which was borrowed through
German
German(s) may refer to:
* Germany (of or related to)
** Germania (historical use)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizens of Germany, see also German nationality law
**Ge ...
''isomerisch''Merriam-Webster"isomeric"
online dictionary entry. Accessed on 2020-08-26 from
Swedish
Swedish or ' may refer to:
Anything from or related to Sweden, a country in Northern Europe. Or, specifically:
* Swedish language, a North Germanic language spoken primarily in Sweden and Finland
** Swedish alphabet, the official alphabet used by ...
; which in turn was coined from Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
ἰσόμερoς , with roots = "equal", = "part".
Structural isomers
Structural isomers have the same number of atoms of each element (hence the samemolecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
), but the atoms are connected in distinct ways.
Example:
For example, there are three distinct compounds with the molecular formula The first two isomers shown of
The first two isomers shown of propanol
There are two isomers of propanol.
* 1-Propanol, ''n''-propanol, or propan-1-ol : CH3CH2CH2OH, the most common meaning
*2-Propanol, Isopropyl alcohol, isopropanol, or propan-2-ol : (CH3)2CHOH
See also
* Propanal (propionaldehyde) differs in sp ...
s, that is, alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
derived from propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used a ...
. Both have a chain of three carbon atoms connected by single bonds, with the remaining carbon valences being filled by seven hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
atoms and by a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
atom bound to a hydrogen atom. These two isomers differ on which carbon the hydroxyl is bound to: either to an extremity of the carbon chain propan-1-ol
Propan-1-ol (also propanol, n-propyl alcohol) is a primary alcohol with the formula and sometimes represented as PrOH or ''n''-PrOH. It is a colorless liquid and an isomer of 2-propanol. It is formed naturally in small amounts during many ferme ...
(1-propanol, ''n''-propyl alcohol, ''n''-propanol; I) or to the middle carbon propan-2-ol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
(2-propanol, isopropyl alcohol, isopropanol; II). These can be described by the condensed structural formulas ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
methoxyethane
Methoxyethane, also known as ethyl methyl ether, is a colorless gaseous ether. Unlike the related dimethyl ether and diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviat ...
(ethyl-methyl-ether; III). Unlike the other two, it has the oxygen atom connected to two carbons, and all eight hydrogens bonded directly to carbons. It can be described by the condensed formula double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s or functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s, occur on a "parent" molecule (propane, in that case).
Example:
There are also three structural isomers of thehydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ex ...
propadiene
Propadiene () or allene () is the organic compound with the formula . It is the simplest allene, i.e. a compound with two adjacent carbon double bonds. As a constituent of MAPP gas, it has been used as a fuel for specialized welding.
Production ...
or allene; I) the carbons are connected by two double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s, while in the other propyne or methylacetylene, II) they are connected by a single bond and a triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
. In the third isomer (cyclopropene
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental st ...
; III) the three carbons are connected into a ring by two single bonds and a double bond. In all three, the remaining valences of the carbon atoms are satisfied by the four hydrogens.
Again, note that there is only one structural isomer with a triple bond, because the other possible placement of that bond is just drawing the three carbons in a different order. For the same reason, there is only one cyclopropene, not three.
Tautomers
Tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
s are structural isomers which readily interconvert, so that two or more species co-exist in equilibrium such as
keto-enol tautomerism
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
and the equilibrium between neutral and zwitterionic
In chemistry, a zwitterion ( ; ), also called an inner salt or dipolar ion, is a molecule that contains an equal number of positively- and negatively-charged functional groups.
: With amino acids, for example, in solution a chemical equilibrium wil ...
forms of an amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
.
Resonance forms
The structure of some molecules is sometimes described as aresonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
between several apparently different structural isomers. The classical example is 1,2-dimethylbenzene (''o''-xylene), which is often described as a mix of the two apparently distinct structural isomers:
However, neither of these two structures describes a real compound; they are fictions devised as a way to describe (by their "averaging" or "resonance") the actual delocalized bonding of ''o''-xylene, which is the single isomer of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
core and two methyl groups in adjacent positions.
Stereoisomers
Stereoisomers have the same atoms or isotopes connected by bonds of the same type, but differ in their shapes – the relative positions of those atoms in space – apart from rotations andtranslations
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
.
In theory, one can imagine any arrangement in space of the atoms of a molecule or ion to be gradually changed to any other arrangement in infinitely many ways, by moving each atom along an appropriate path. However, changes in the positions of atoms will generally change the internal energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat a ...
of a molecule, which is determined by the angles between bonds in each atom and by the distances between atoms (whether they are bonded or not).
A conformational isomer
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a mo ...
is an arrangement of the atoms of the molecule or ion for which the internal energy is a local minimum
In mathematical analysis, the maxima and minima (the respective plurals of maximum and minimum) of a function, known collectively as extrema (the plural of extremum), are the largest and smallest value of the function, either within a given ran ...
; that is, an arrangement such that any small changes in the positions of the atoms will increase the internal energy, and hence result in forces that tend to push the atoms back to the original positions. Changing the shape of the molecule from such an energy minimum energy barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
: the amount that must be temporarily added to the internal energy of the molecule in order to go through all the intermediate conformations along the "easiest" path (the one that minimizes that amount).
 A classic example of conformational isomerism is
A classic example of conformational isomerism is cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
. Alkanes
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which ...
generally have minimum energy when the chair
A chair is a type of seat, typically designed for one person and consisting of one or more legs, a flat or slightly angled seat and a back-rest. They may be made of wood, metal, or synthetic materials, and may be padded or upholstered in vario ...
(with the carbons alternately above and below their mean plane) and boat
A boat is a watercraft of a large range of types and sizes, but generally smaller than a ship, which is distinguished by its larger size, shape, cargo or passenger capacity, or its ability to carry boats.
Small boats are typically found on inl ...
(with two opposite carbons above the plane, and the other four below it).
If the energy barrier between two conformational isomers is low enough, it may be overcome by the random inputs of thermal energy
The term "thermal energy" is used loosely in various contexts in physics and engineering. It can refer to several different well-defined physical concepts. These include the internal energy or enthalpy of a body of matter and radiation; heat, d ...
that the molecule gets from interactions with the environment or from its own vibrations
Vibration is a mechanical phenomenon whereby oscillations occur about an equilibrium point. The word comes from Latin ''vibrationem'' ("shaking, brandishing"). The oscillations may be periodic, such as the motion of a pendulum—or random, such ...
. In that case, the two isomers may as well be considered a single isomer, depending on the temperature and the context. For example, the two conformations of cyclohexane convert to each other quite rapidly at room temperature (in the liquid state), so that they are usually treated as a single isomer in chemistry.
In some cases, the barrier can be crossed by quantum tunneling
In physics, a quantum (plural quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a physical property can be "quantized" is referred to as "the hypothesis of quantizati ...
of the atoms themselves. This last phenomenon prevents the separation of stereoisomers of fluorochloroamine hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
picosecond
A picosecond (abbreviated as ps) is a unit of time in the International System of Units (SI) equal to 10−12 or (one trillionth) of a second. That is one trillionth, or one millionth of one millionth of a second, or 0.000 000 000 ...
s even at very low temperatures.Rowena Ball and John Brindley (2016): "The life story of hydrogen peroxide III: Chirality and physical effects at the dawn of life". ''Origins of Life and Evolution of Biospheres'', volume 46, pages 81–93
Conversely, the energy barrier may be so high that the easiest way to overcome it would require temporarily breaking and then reforming one or more bonds of the molecule. In that case, the two isomers usually are stable enough to be isolated and treated as distinct substances. These isomers are then said to be different configurational isomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms i ...
s or "configurations" of the molecule, not just two different conformations.Vallurupalli S. R. Rao (1998):Methods of conformational analysis
. Chapter 2 in ''Conformation of Carbohydrates''. 409 pages. (However, one should be aware that the terms "conformation" and "configuration" are largely synonymous outside of chemistry, and their distinction may be controversial even among chemists.Anatoly M Belostotskii (2015):
Conformer and conformation
, chapter 2 of ''Conformational Concept For Synthetic Chemist's Use: Principles And in Lab Exploitation''. 580 pages. .) Interactions with other molecules of the same or different compounds (for example, through
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s) can significantly change the energy of conformations of a molecule. Therefore, the possible isomers of a compound in solution or in its liquid and solid phases many be very different from those of an isolated molecule in vacuum. Even in the gas phase, some compounds like acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
will exist mostly in the form of dimers or larger groups of molecules, whose configurations may be different from those of the isolated molecule.
Enantiomers
Two compounds are said to beenantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
s if their molecules are mirror images of each other, that cannot be made to coincide only by rotations or translations – like a left hand and a right hand. The two shapes are said to be chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
.
A classical example is bromochlorofluoromethane (chlorofluoromethane
Chlorofluoromethane or Freon 31 is the hydrochlorofluorocarbon (HCFC) with the formula CH2ClF. It is a colorless, odorless, flammable gas.
Uses
Pyrolysis of a mixture of dichlorofluoromethane and chlorofluoromethane gives hexafluorobenzene:
:3 C ...
right-hand rule
In mathematics and physics, the right-hand rule is a common mnemonic for understanding orientation of axes in three-dimensional space. It is also a convenient method for quickly finding the direction of a cross-product of 2 vectors.
Most of th ...
. This type of isomerism is called axial isomer
Axial may refer to:
* one of the Anatomical terms of location#Other directional terms, anatomical directions describing relationships in an animal body
* In geometry:
:* a geometric term of location
:* an axis of rotation
* In chemistry, referring ...
ism.
Enantiomers behave identically in chemical reactions, except when reacted with chiral compounds or in the presence of chiral catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, such as most enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s. For this latter reason, the two enantiomers of most chiral compounds usually have markedly different effects and roles in living organisms. In biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
and food science
Food science is the basic science and applied science of food; its scope starts at overlap with agricultural science and nutritional science and leads through the scientific aspects of food safety and food processing, informing the developm ...
, the two enantiomers of a chiral molecule – such as glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using ...
– are usually identified, and treated as very different substances.
Each enantiomer of a chiral compound typically rotates the plane of polarized light
Polarization (also polarisation) is a property applying to transverse waves that specifies the geometrical orientation of the oscillations. In a transverse wave, the direction of the oscillation is perpendicular to the direction of motion of the ...
that passes through it. The rotation has the same magnitude but opposite senses for the two isomers, and can be a useful way of distinguishing and measuring their concentration in a solution. For this reason, enantiomers were formerly called "optical isomers". However, this term is ambiguous and is discouraged by the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
.
Stereoisomers that are not enantiomers are called diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
s. Some diastereomers may contain chiral center
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups ...
, some not.
Some enantiomer pairs (such as those of ''trans''-cyclooctene) can be interconverted by internal motions that change bond lengths and angles only slightly. Other pairs (such as CHFClBr) cannot be interconverted without breaking bonds, and therefore are different configurations.
Cis-trans isomerism
A double bond between two carbon atoms forces the remaining four bonds (if they are single) to lie on the same plane, perpendicular to the plane of the bond as defined by its π orbital. If the two bonds on each carbon connect to different atoms, two distinct conformations are possible, that differ from each other by a twist of 180 degrees of one of the carbons about the double bond. The classical example is dichloroetheneIUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
recommended nomenclature. Conversion between these two forms usually requires temporarily breaking bonds (or turning the double bond into a single bond), so the two are considered different configurations of the molecule.
More generally, ''cis''–''trans'' isomerism (formerly called "geometric isomerism") occurs in molecules where the relative orientation of two distinguishable functional groups is restricted by a somewhat rigid framework of other atoms.
For example, in the cyclic alcohol inositol
Inositol, or more precisely ''myo''-inositol, is a carbocyclic sugar that is abundant in the brain and other mammalian tissues; it mediates cell signal transduction in response to a variety of hormones, neurotransmitters, and growth factors and ...
coordination compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
s, such as square planar
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corne ...
octahedral
In geometry, an octahedron (plural: octahedra, octahedrons) is a polyhedron with eight faces. The term is most commonly used to refer to the regular octahedron, a Platonic solid composed of eight equilateral triangles, four of which meet at ea ...
Centers with non-equivalent bonds
More generally, atoms or atom groups that can form three or more non-equivalent single bonds (such as thetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s in coordination compounds) may give rise to multiple stereoisomers when different atoms or groups are attached at those positions. The same is true if a center with six or more equivalent bonds has two or more substituents.
For instance, in the compound phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
atom to the five halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s have approximately trigonal bipyramidal geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identi ...
. Thus two stereoisomers with that formula are possible, depending on whether the chlorine atom occupies one of the two "axial" positions, or one of the three "equatorial" positions.
For the compound octahedral geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The oc ...
, has at least two facial–meridional isomers, depending on whether the three Rotamers and atropisomers
Two parts of a molecule that are connected by just one single bond can rotate about that bond. While the bond itself is indifferent to that rotation, attractions and repulsions between the atoms in the two parts normally cause the energy of the whole molecule to vary (and possibly also the two parts to deform) depending on the relative angle of rotation φ between the two parts. Then there will be one or more special values of φ for which the energy is at a local minimum. The corresponding conformations of the molecule are called rotational isomers orrotamer
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a mole ...
s.
Thus, for example, in an ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petr ...
molecule methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
groups can independently rotate about the 1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl ...
(butane
Butane () or ''n''-butane is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that quickly vaporizes at room temperature. The name but ...
is similar, but with sightly lower ''gauche'' energies and barriers.
If the two parts of the molecule connected by a single bond are bulky or charged, the energy barriers may be much higher. For example, in the compound biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
– two phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
groups connected by a single bond – the repulsion between hydrogen atoms closest to the central single bond gives the fully planar conformation, with the two rings on the same plane, a higher energy than conformations where the two rings are skewed. In the gas phase, the molecule has therefore at least two rotamers, with the ring planes twisted by ±47°, which are mirror images of each other. The barrier between them is rather low (~8 kJ/mol).A. T. H. Lenstra, C. Van Alsenoy, K. Verhulst and H. J. Geise (1994): "Solids modelled by crystal field ab initio methods. 5. The phase transitions in biphenyl from a molecular point of view". ''Acta Crystallographica Section B'', volume B50, pages 96-106. This steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
effect is more pronounced when those four hydrogens are replaced by larger atoms or groups, like chlorines or carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s. If the barrier is high enough for the two rotamers to be separated as stable compounds at room temperature, they are called atropisomers.
Topoisomers
Large molecules may have isomers that differ by thetopology
In mathematics, topology (from the Greek language, Greek words , and ) is concerned with the properties of a mathematical object, geometric object that are preserved under Continuous function, continuous Deformation theory, deformations, such ...
of their overall arrangement in space, even if there is no specific geometric constraint that separate them. For example, long chains may be twisted to form topologically distinct knot
A knot is an intentional complication in cordage which may be practical or decorative, or both. Practical knots are classified by function, including hitches, bends, loop knots, and splices: a ''hitch'' fastens a rope to another object; a ' ...
s, with interconversion prevented by bulky substituents or cycle closing (as in circular DNA and RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
plasmid
A plasmid is a small, extrachromosomal DNA molecule within a cell that is physically separated from chromosomal DNA and can replicate independently. They are most commonly found as small circular, double-stranded DNA molecules in bacteria; how ...
s). Some knots may come in mirror-image enantiomer pairs. Such forms are called topological isomers or topoisomer Topoisomers or topological isomers are molecules with the same chemical formula and stereochemical bond connectivities but different topologies. Examples of molecules for which there exist topoisomers include DNA, which can form knots, and catenan ...
s.
Also, two or more such molecules may be bound together in a catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be se ...
by such topological linkages, even if there is no chemical bond between them. If the molecules are large enough, the linking may occur in multiple topologically distinct ways, constituting different isomers. Cage compound
In host–guest chemistry, an inclusion compound (also known as an inclusion complex) is a chemical complex in which one chemical compound (the "host") has a cavity into which a "guest" compound can be accommodated. The interaction between the h ...
s, such as helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
enclosed in dodecahedrane
Dodecahedrane is a chemical compound, a hydrocarbon with formula , whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound ...
(He@) and carbon peapods, are a similar type of topological isomerism involving molecules with large internal voids with restricted or no openings.Takahiro Iwamoto, Yoshiki Watanabe, Tatsuya Sadahiro, Takeharu Haino, and Shigeru Yamago (2011): "Size-selective encapsulation of C60 by 0ycloparaphenylene: Formation of the shortest fullerene-peapod". ''Angewandte Chemie International Edition'', volume 50, issue 36, pages 8342–8344.
Isotopes and spin
Isotopomers
Different isotopes of the same element can be considered as different kinds of atoms when enumerating isomers of a molecule or ion. The replacement of one or more atoms by their isotopes can create multiple structural isomers and/or stereoisomers from a single isomer. For example, replacing two atoms of commonhydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
(deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen (the other being Hydrogen atom, protium, or hydrogen-1). The atomic nucleus, nucleus of a deuterium ato ...
(ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petr ...
molecule yields two distinct structural isomers, depending on whether the substitutions are both on the same carbon (1,1-dideuteroethane, chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
instead of deuterium. The two molecules do not interconvert easily and have different properties, such as their microwave spectrum.Eizi Hirota (2012): "Microwave spectroscopy of isotope-substituted non-polar molecules". Chapter 5 in ''Molecular Spectroscopy: Modern Research'', volume 3. 466 pages.
Another example would be substituting one atom of deuterium for one of the hydrogens in chlorofluoromethane
Chlorofluoromethane or Freon 31 is the hydrochlorofluorocarbon (HCFC) with the formula CH2ClF. It is a colorless, odorless, flammable gas.
Uses
Pyrolysis of a mixture of dichlorofluoromethane and chlorofluoromethane gives hexafluorobenzene:
:3 C ...
(isotopomer
Isotopomers or isotopic isomers are isomers with isotopic atoms, having the same number of each isotope of each element but differing in their positions. The result is that the molecules are either constitutional isomers or stereoisomers solely ...
s or isotopic isomers. In the above two examples if all Spin isomers
Another type of isomerism based on nuclear properties is spin isomerism, where molecules differ only in the relative spin magnetic quantum numbers ms of the constituent atomic nuclei. This phenomenon is significant for molecular hydrogen, which can be partially separated into two long-lived states described as spin isomers or nuclear spin isomers: parahydrogen, with the spins of the two nuclei pointing in opposite directions, and orthohydrogen, where the spins point in the same direction.Isomerization
Isomerization is the process by which one molecule is transformed into another molecule that has exactly the same atoms, but the atoms are rearranged. In some molecules and under some conditions, isomerization occurs spontaneously. Many isomers are equal or roughly equal inbond energy
In chemistry, bond energy (''BE''), also called the mean bond enthalpy or average bond enthalpy is the measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually at ...
, and so exist in roughly equal amounts, provided that they can interconvert relatively freely, that is the energy barrier between the two isomers is not too high. When the isomerization occurs intramolecularly, it is considered a rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another ...
.
An example of an organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
isomerization is the production of decaphenylferrocene, Ph5)2Fe.html" ;"title="phenyl.html" ;"title="η5-C5Ph5)2Fe">phenyl.html"_;"title="η5-C5phenyl">Ph5)2Fefrom_its_linkage_isomerism.html" "title="phenyl">Ph5)2Fe">phenyl.html" ;"title="η5-C5phenyl">Ph5)2Fefrom its linkage isomerism">linkage isomer
In chemistry, linkage isomerism or ambidentate isomerism is a form of isomerism in which certain coordination compounds have the same composition but differ in their metal atom's connectivity to a ligand.
Typical ligands that give rise to linkage ...
.

Topoisomerase
DNA topoisomerases (or topoisomerases) are enzymes that catalyze changes in the topological state of DNA, interconverting relaxed and supercoiled forms, linked (catenated) and unlinked species, and knotted and unknotted DNA. Topological issues i ...
s are enzymes that can cut and reform circular DNA and thus change its topology.
Medicinal chemistry
Isomers having distinct biological properties are common; for example, the placement ofmethyl group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many ...
s. In substituted xanthine
Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base (genetics), base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, incl ...
s, theobromine
Theobromine, also known as xantheose, is the principal alkaloid of '' Theobroma cacao'' (cacao plant). Theobromine is slightly water-soluble (330 mg/L) with a bitter taste. In industry, theobromine is used as an additive and precursor to ...
, found in chocolate, is a vasodilator
Vasodilation is the widening of blood vessels. It results from relaxation of smooth muscle cells within the vessel walls, in particular in the large veins, large arteries, and smaller arterioles. The process is the opposite of vasoconstriction, ...
with some effects in common with caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine t ...
; but, if one of the two methyl groups is moved to a different position on the two-ring core, the isomer is theophylline
Theophylline, also known as 1,3-dimethylxanthine, is a phosphodiesterase inhibiting drug used in therapy for respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma under a variety of brand names. As a member of the ...
, which has a variety of effects, including bronchodilation
A bronchodilator or broncholytic (although the latter occasionally includes secretory inhibition as well) is a substance that dilates the bronchi and bronchioles, decreasing resistance in the respiratory airway and increasing airflow to the lun ...
and anti-inflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs remedy pain by reducing inflammation as o ...
action. Another example of this occurs in the phenethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amin ...
-based stimulant drugs. Phentermine
Phentermine ( phenyl- tertiary-butyl amine), with several brand names including Ionamin and Sentis, is a medication used together with diet and exercise to treat obesity. It is taken by mouth for up to a few weeks at a time, after which the ben ...
is a non-chiral compound with a weaker effect than that of amphetamine
Amphetamine (contracted from alpha- methylphenethylamine) is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. It is also commonly used ...
. It is used as an appetite-reducing medication and has mild or no stimulant properties. However, an alternate atomic arrangement gives dextromethamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug and less commonly as a second-line treatment for attention deficit hyperactivity disorder and obesity. Methamphe ...
, which is a stronger stimulant than amphetamine.
In medicinal chemistry
Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with designing and developing pharmaceutical drugs. Medicinal chemistry involves the identification, synthesis and developm ...
and biochemistry, enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
s are a special concern because they may possess distinct biological activity
In pharmacology, biological activity or pharmacological activity describes the beneficial or adverse effects of a drug on living matter. When a drug is a complex chemical mixture, this activity is exerted by the substance's active ingredient or ...
. Many preparative procedures afford a mixture of equal amounts of both enantiomeric forms. In some cases, the enantiomers are separated by chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a ...
using chiral stationary phases. They may also be separated through the formation of diastereomeric salts. In other cases, enantioselective synthesis have been developed.
As an inorganic example, cisplatin
Cisplatin is a chemotherapy medication used to treat a number of cancers. These include testicular cancer, ovarian cancer, cervical cancer, breast cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelioma, br ...
(see structure above) is an important drug used in cancer chemotherapy, whereas the trans isomer (transplatin) has no useful pharmacological activity.
History
Isomerism was first observed in 1827, whenFriedrich Wöhler
Friedrich Wöhler () FRS(For) HonFRSE (31 July 180023 September 1882) was a German chemist known for his work in inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the firs ...
prepared silver cyanate
Silver cyanate is the cyanate salt of silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transit ...
and discovered that, although its elemental composition of silver fulminate
Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.
Silver fulminate is a primary explosive, but has limited use as such due to its extreme sensitivity to impact, heat, pressure, and electricity. The compound becomes pr ...
(prepared by Justus von Liebig
Justus Freiherr von Liebig (12 May 1803 – 20 April 1873) was a German scientist who made major contributions to agricultural and biological chemistry, and is considered one of the principal founders of organic chemistry. As a professor at t ...
the previous year), its properties were distinct. This finding challenged the prevailing chemical understanding of the time, which held that chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s could be distinct only when their elemental compositions differ. (We now know that the bonding structures of fulminate
Fulminates are chemical compounds which include the fulminate ion (). The fulminate ion is a pseudohalic ion because its charge and reactivity are similar to those of the halogens. Due to the instability of the ion, fulminate salts are friction ...
and cyanate
Cyanate is an anion with the structural formula , usually written . It also refers to any salt containing it, such as ammonium cyanate.
It is an isomer of the much less stable fulminate anion .William R. Martin and David W. Ball (2019): "Small ...
can be approximately described as urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
has the same atomic composition (ammonium cyanate
Ammonium cyanate is an inorganic compound with the formula . It is a colorless, solid salt.
Structure and reactions
The structure of this salt was verified by X-ray crystallography. The respective C–O and C–N distances are 1.174(8) and 1.1 ...
. (Their structures are now known to be H+4 H4, H04, or H-4 may refer to:
Science and mathematics
* ATC code H04 ''Pancreatic hormones'', a subgroup of the Anatomical Therapeutic Chemical Classification System
* Histamine H4 receptor, a human gene
* Histone H4, a protein involved in the s ...
=C=N^ -Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be on ...
introduced the term ''isomerism'' to describe the phenomenon.Jac. Berzelius (1830):Om sammansättningen af vinsyra och drufsyra (John's säure aus den Voghesen), om blyoxidens atomvigt, samt allmänna anmärkningar om sådana kroppar som hafva lika sammansättning, men skiljaktiga egenskaper
("On the composition of tartaric acid and racemic acid (John's acid of the Vosges), on the molecular weight of lead oxide, together with general observations on those bodies that have the same composition but distinct properties"). ''Kongliga Svenska Vetenskaps Academiens Handling'' (''Transactions of the Royal Swedish Science Academy''), volume 49, pages 49–80J. J. Berzelius (1831):
Über die Zusammensetzung der Weinsäure und Traubensäure (John's säure aus den Voghesen), über das Atomengewicht des Bleioxyds, nebst allgemeinen Bemerkungen über solche Körper, die gleiche Zusammensetzung, aber ungleiche Eigenschaften besitzen
. ''Annalen der Physik und Chemie'', volume 19, pages 305–335J. J. Berzelius (1831):
Composition de l'acide tartarique et de l'acide racémique (traubensäure); poids atomique de l'oxide de plomb, et remarques générals sur les corps qui ont la même composition, et possèdent des proprietés différentes
. ''Annales de Chimie et de Physique'', volume 46, pages 113–147. In 1848,
Louis Pasteur
Louis Pasteur (, ; 27 December 1822 – 28 September 1895) was a French chemist and microbiologist renowned for his discoveries of the principles of vaccination, microbial fermentation and pasteurization, the latter of which was named afte ...
observed that tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally i ...
crystals came into two kinds of shapes that were mirror images of each other. Separating the crystals by hand, he obtained two version of tartaric acid, each of which would crystallize in only one of the two shapes, and rotated the plane of polarized light to the same degree but in opposite directions.L. Pasteur (1848"Sur les relations qui peuvent exister entre la forme cristalline, la composition chimique et le sens de la polarisation rotatoire"
("On the relations that can exist between crystalline form, chemical composition, and the sense of rotary polarization"), ''Annales de Chimie et de Physique'', 3rd series, volume 24, issue 6, pages 442–459.
See also
*Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotation (geometry), rotations, translation (geometry), translations, and some Conformational isomerism, conformational ch ...
* Cis-trans isomerism
* Cyclohexane conformation
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are ...
* Descriptor (chemistry)
A descriptor is in chemical nomenclature a prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some listed descriptors are only of historical interest and should not be us ...
* Electromerism Electromerism is a type of isomerism between a pair of molecules (electromers, electro-isomers) differing in the way electrons are distributed among the atoms and the connecting chemical bonds. In some literature electromerism is equated to valenc ...
* Isomery (botany)
* Ligand isomerism
* Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state, higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited ...
* Stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups c ...
* Structural isomerism
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metam ...
* Tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
* Vitamer
Vitamins occur in a variety of related forms known as vitamers. A vitamer () of a particular vitamin is one of several related compounds that performs the functions of said vitamin and prevents the symptoms of deficiency of said vitamin.
Early r ...
References
External links
{{Commons category, Isomerism Isomerism 1827 introductions ga:Isiméir