5-Fluorowillardiine on:
[Wikipedia]
[Google]
[Amazon]
5-Fluorowillardiine is a selective agonist for the AMPA receptor, with only limited effects at the
 5-fluorowillardiine is derived from the nitrogenous base
5-fluorowillardiine is derived from the nitrogenous base
MeSHName: 5-Fluorowillardiine
{{DEFAULTSORT:Fluorowillardiine, 5- AMPA receptor agonists Organofluorides Amino acids Pyrimidinediones
kainate receptor
Kainate receptors, or kainic acid receptors (KARs), are ionotropic receptors that respond to the neurotransmitter glutamate. They were first identified as a distinct receptor type through their selective activation by the agonist kainate, a dru ...
. It is an excitotoxic
In excitotoxicity, nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as glutamate become pathologically high, resulting in excessive stimulation of receptors. For example, when glutamate re ...
neurotoxin when used ''in vivo'' and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors ''in vitro''.
It is structurally similar to the compound willardiine, which is also an agonist for the AMPA and kainate receptors. Willardiine occurs naturally in Mariosousa willardiana and Acacia sensu lato
''Acacia s.l.'' (pronounced or ), known commonly as mimosa, acacia, thorntree or wattle, is a polyphyletic genus of shrubs and trees belonging to the subfamily Mimosoideae of the family Fabaceae. It was described by the Swedish botanist Carl ...
.
The name is unusual as it has two successive i's. This is not a typo
A typographical error (often shortened to typo), also called a misprint, is a mistake (such as a spelling mistake) made in the typing of printed (or electronic) material. Historically, this referred to mistakes in manual type-setting (typography) ...
.
Toxicity
(S)-5-Fluorowillardiine activity has been studied ''in vitro'' in a variety of neural tissues. In mouse embryo hippocampal neurons, it was found to desensitize AMPA/kainate receptors with anEC50
]
Half maximal effective concentration (EC50) is a measure of the concentration of a drug, antibody or toxicant which induces a Stimulus%E2%80%93response_model, response halfway between the baseline and maximum after a specified exposure time. Mo ...
of 1.5 μM –— 7 times more potent than racemic AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, better known as AMPA, is a compound that is a specific agonist for the AMPA receptor, where it mimics the effects of the neurotransmitter glutamate.
There are several types of glutamatergic ...
(EC50 of 11 μM). In another study, (S)-5-Fluorowillardiine showed biphasic dose-dependent neurotoxicity in cultural rodent cortical neurons, with EC50 vales of 0.70 and 170 μM.
While ''in vivo'' research is sparse, a study in 5-day-old mice injected with the closely related AMPA/kainate agonist (S)-5-Bromowillardiine showed cortical and white matter damage. AMPA antagonists reduced the extent of the damage in a dose-dependent fashion.
Applications in research
Radiolabeled 5-fluorowillardiine has been used to study the distribution of ionotropic glutamate receptors in rodent brains. It has also been used to evaluate the effects of various allosteric modulators of the AMPA receptor.Chemistry
Structure and activity
 5-fluorowillardiine is derived from the nitrogenous base
5-fluorowillardiine is derived from the nitrogenous base uracil
Uracil () (symbol U or Ura) is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced b ...
found in RNA. It is one member of a family of willardiine compounds, which share uracil or a substituted uracil as an amino acid side chain.
5-Fluorowillardiine exists as two distinct isomers
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
...
:
* (2''R'') or D
* (2''S'') or L
The particularly high affinity of 5-fluorowillardiine for the AMPA receptor is attributed to its fluorine substituent at the 5-position of the ring, which is electron-withdrawing and small enough to not interfere with binding. By contrast, related willardiine derivatives with larger nonpolar electron withdrawing groups exhibit greater affinity for kainate receptors than 5-fluorowillardiine, and less affinity for AMPA receptors.
The binding of 5-fluorowillardiine to the AMPA receptor is driven by entropy when its ring is uncharged. When the ring is deprotonated and has a negative charge, a favorable change in enthalpy primarily drives binding. Because the pKa values of halogenated willardiine derivates are approximately 8 (7.98 for 5-Fluorowillardiine), binding is mostly driven by an increase in entropy at physiological pH.
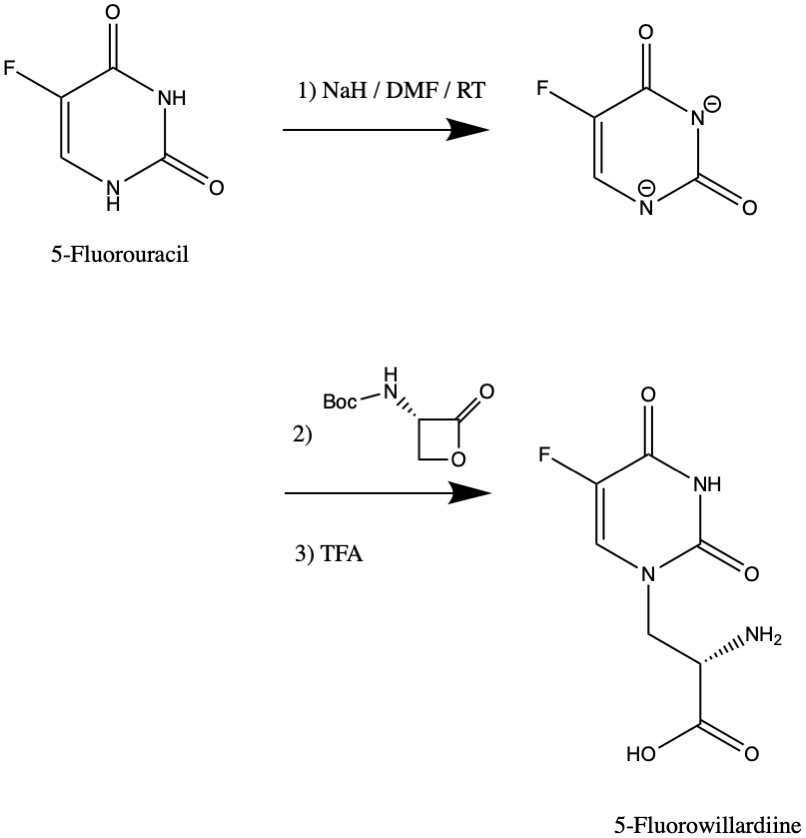
Synthesis
The synthesis of 5-Fluorowillardiine may be achieved by using5-Fluorouracil
Fluorouracil (5-FU), sold under the brand name Adrucil among others, is a cytotoxic chemotherapy medication used to treat cancer. By intravenous injection it is used for treatment of colorectal cancer, oesophageal cancer, stomach cancer, pan ...
as a nucleophile to open a specialized lactone in an SN2 reaction
The SN2 reaction is a type of reaction mechanism that is common in organic chemistry. In this mechanism, one bond is broken and one bond is formed in a concerted way, i.e., in one step. The name SN2 refers to the Hughes-Ingold symbol of the m ...
. Another straightforward approach is to perform a Strecker amino acid synthesis.
References
External links
MeSHName: 5-Fluorowillardiine
{{DEFAULTSORT:Fluorowillardiine, 5- AMPA receptor agonists Organofluorides Amino acids Pyrimidinediones