Sulfate-reducing microorganisms on:
[Wikipedia]
[Google]
[Amazon]
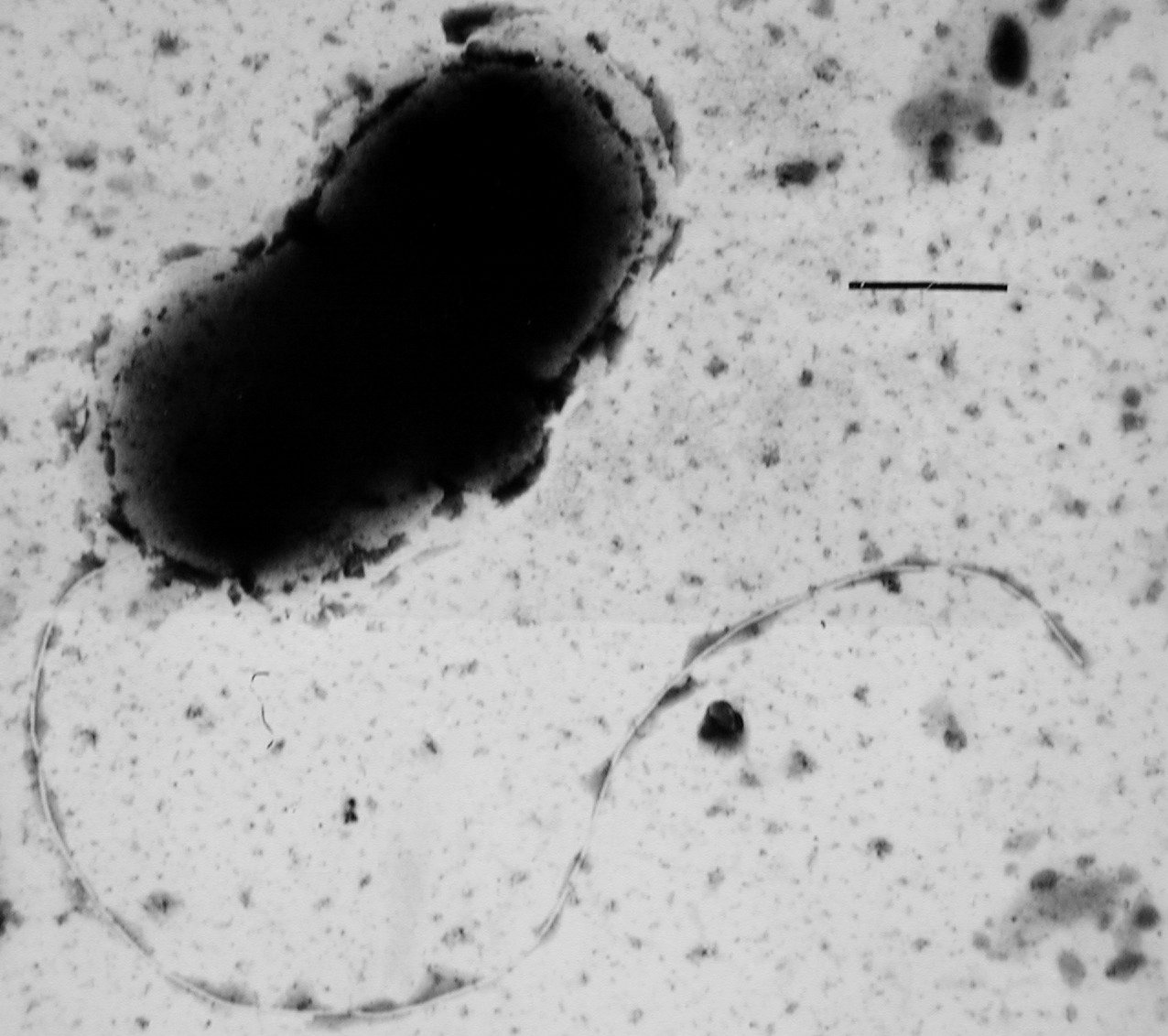 Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing
 The toxic
The toxic
 The enzyme dissimilatory (bi)sulfite reductase, ''dsrAB'' (EC 1.8.99.5), that catalyzes the last step of dissimilatory sulfate reduction, is the functional gene most used as a molecular marker to detect the presence of sulfate-reducing microorganisms.
The enzyme dissimilatory (bi)sulfite reductase, ''dsrAB'' (EC 1.8.99.5), that catalyzes the last step of dissimilatory sulfate reduction, is the functional gene most used as a molecular marker to detect the presence of sulfate-reducing microorganisms.
Oldest Water on Earth Found Deep Within the Canadian Shield
December 14, 2016, Maggie Romuld
'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory
Garnet S. Lollar, Oliver Warr, Jon Telling, Magdalena R. Osburn & Barbara Sherwood Lollar, Received 15 Jan 2019, Accepted 01 Jul 2019, Published online: 18 Jul 2019.
Deep fracture fluids isolated in the crust since the Precambrian era
G. Holland, B. Sherwood Lollar, L. Li, G. Lacrampe-Couloume, G. F. Slater & C. J. Ballentine, Nature volume 497, pages 357–360 (16 May 2013)
Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks
by L. Li, B. A. Wing, T. H. Bui, J. M. McDermott, G. F. Slater, S. Wei, G. Lacrampe-Couloume & B. Sherwood Lollar October 27, 2016. Nature Communications volume 7, Article number: 13252 (2016.)
Earth's mysterious 'deep biosphere' may harbor millions of undiscovered species
By Brandon Specktor, Live Science, December 11, 2018, published online at nbcnews.com. {{DEFAULTSORT:Sulfate-Reducing Bacteria Bacteria Martinus Beijerinck Extremophiles Environmental microbiology Ecology Geomicrobiology Microbial growth and nutrition Thermodesulfobacteriota
bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
(SRB) and sulfate-reducing archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
(SRA), both of which can perform anaerobic respiration utilizing sulfate () as terminal electron acceptor, reducing it to hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
(H2S). Therefore, these sulfidogenic microorganism
A microorganism, or microbe, is an organism of microscopic scale, microscopic size, which may exist in its unicellular organism, single-celled form or as a Colony (biology)#Microbial colonies, colony of cells. The possible existence of unseen ...
s "breathe" sulfate rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
(H2O) in aerobic respiration.
Most sulfate-reducing microorganisms can also reduce some other oxidized inorganic sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
compounds, such as sulfite (), dithionite (), thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
(), trithionate (), tetrathionate
The tetrathionate anion, , is a sulfur oxyanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5. Alternatively, the compound can be vi ...
(), elemental sulfur (S8), and polysulfides (). Other than sulfate reduction, some sulfate-reducing microorganisms are also capable of other reactions like disproportionation of sulfur compounds. Depending on the context, "sulfate-reducing microorganisms" can be used in a broader sense (including all species that can reduce any of these sulfur compounds) or in a narrower sense (including only species that reduce sulfate, and excluding strict thiosulfate and sulfur reducers, for example).
Sulfate-reducing microorganisms can be traced back to 3.5 billion years ago and are considered to be among the oldest forms of microbes, having contributed to the sulfur cycle soon after life emerged on Earth.
Many organisms reduce small amounts of sulfates in order to synthesize sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
-containing cell components; this is known as ''assimilatory sulfate reduction''. By contrast, the sulfate-reducing microorganisms considered here reduce sulfate in large amounts to obtain energy and expel the resulting sulfide as waste; this is known as dissimilatory sulfate reduction. They use sulfate as the terminal electron acceptor of their electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
. Most of them are anaerobes; however, there are examples of sulfate-reducing microorganisms that are tolerant of oxygen, and some of them can even perform aerobic respiration.
No growth is observed when oxygen is used as the electron acceptor.
"
In addition, there are sulfate-reducing microorganisms that can also reduce other electron acceptors, such as fumarate, nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
(), nitrite (), ferric iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
(Fe3+), and dimethyl sulfoxide (DMSO).
In terms of electron donor, this group contains both organotrophs and lithotrophs. The organotrophs oxidize organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s, such as carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s, organic acids (such as formate, lactate, acetate, propionate, and butyrate), alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s ( methanol and ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
), aliphatic hydrocarbons (including methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
), and aromatic hydrocarbons (benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
, ethylbenzene, and xylene). The lithotrophs oxidize molecular hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
(H2), for which they compete with methanogens and acetogens in anaerobic conditions. Some sulfate-reducing microorganisms can directly use metallic iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
(Fe0, also known as zerovalent iron, or ZVI) as an electron donor, oxidizing it to ferrous iron (Fe2+).
Ecological importance and markers
Sulfate occurs widely in seawater, sediment, and water rich in decaying organic material. Sulfate is also found in more extreme environments such as hydrothermal vents, acid mine drainage sites, oil fields, and the deep subsurface, including the world's oldest isolated ground water. Sulfate-reducing microorganisms are common in anaerobic environments where they aid in the degradation of organic materials. In these anaerobic environments, fermenting bacteria extract energy from large organic molecules; the resulting smaller compounds such as organic acids andalcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s are further oxidized by acetogens and methanogens and the competing sulfate-reducing microorganisms.
 The toxic
The toxic hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
is a waste product of sulfate-reducing microorganisms; its rotten egg odor is often a marker for the presence of sulfate-reducing microorganisms in nature.
Sulfate-reducing microorganisms are responsible for the sulfurous odors of salt marshes and mud flats. Much of the hydrogen sulfide will react with metal ions in the water to produce metal sulfides. These metal sulfides, such as ferrous sulfide (FeS), are insoluble and often black or brown, leading to the dark color of sludge.
During the Permian–Triassic extinction event (250 million years ago) a severe anoxic event
An anoxic event describes a period wherein large expanses of Earth's oceans were depleted of dissolved oxygen (O2), creating toxic, euxinic ( anoxic and sulfidic) waters. Although anoxic events have not happened for millions of years, the geol ...
seems to have occurred where these forms of bacteria became the dominant force in oceanic ecosystems, producing copious amounts of hydrogen sulfide.
Sulfate-reducing bacteria also generate neurotoxic methylmercury
Methylmercury is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is a bioaccumulative environment ...
as a byproduct of their metabolism, through methylation of inorganic mercury present in their surroundings. They are known to be the dominant source of this bioaccumulative
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance faster than it can be lost or eliminated by catabolism and excretion. ...
form of mercury in aquatic systems.
Uses
Some sulfate-reducing microorganisms can reducehydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s, and they have been used to clean up contaminated soils. Their use has also been proposed for other kinds of contaminations.
Sulfate-reducing microorganisms are considered a possible way to deal with acid mine waters that are produced by other microorganisms.
Problems caused by sulfate-reducing microorganisms
In engineering, sulfate-reducing microorganisms can create problems when metal structures are exposed to sulfate-containing water: Interaction of water and metal creates a layer of molecular hydrogen on the metal surface; sulfate-reducing microorganisms then oxidize the hydrogen while creating hydrogen sulfide, which contributes tocorrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
.
Hydrogen sulfide from sulfate-reducing microorganisms also plays a role in the biogenic sulfide corrosion of concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
. It also occurs in sour crude oil.
Some sulfate-reducing microorganisms play a role in the anaerobic oxidation of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
:
:CH4 + SO42- → HCO3− + HS− + H2O
An important fraction of the methane formed by methanogens below the seabed is oxidized by sulfate-reducing microorganisms in the transition zone separating the methanogenesis from the sulfate reduction activity in the sediments. This process is also considered a major sink for sulfate in marine sediments.
In hydraulic fracturing, fluids are used to frack shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of Clay mineral, clay minerals (hydrous aluminium phyllosilicates, e.g., Kaolinite, kaolin, aluminium, Al2Silicon, Si2Oxygen, O5(hydroxide, OH)4) and tiny f ...
formations to recover methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
( shale gas) and hydrocarbons. Biocide compounds are often added to water to inhibit the microbial activity of sulfate-reducing microorganisms, in order to but not limited to, avoid anaerobic methane oxidation and the generation of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
, ultimately resulting in minimizing potential production loss.
Biochemistry
Before sulfate can be used as an electron acceptor, it must be activated. This is done by the enzyme ATP-sulfurylase, which uses ATP and sulfate to create adenosine 5′-phosphosulfate (APS). APS is subsequently reduced to sulfite and AMP. Sulfite is then further reduced to sulfide, while AMP is turned into ADP using another molecule of ATP. The overall process, thus, involves an investment of two molecules of the energy carrier ATP, which must to be regained from the reduction.Phylogeny
The sulfate-reducing microorganisms have been treated as a phenotypic group, together with the other sulfur-reducing bacteria, for identification purposes. They are found in several different phylogenetic lines. As of 2009, 60 genera containing 220 species of sulfate-reducing bacteria are known. Among the Thermodesulfobacteriota the orders of sulfate-reducing bacteria include Desulfobacterales,Desulfovibrionales
Desulfovibrionales are a taxonomic order of bacteria belonging to the phylum Thermodesulfobacteriota, with four families. They are Gram-negative. The majority are sulfate-reducing, with the exception of '' Lawsonia'' and '' Bilophila''.Garrity ...
, and Syntrophobacterales. This accounts for the largest group of sulfate-reducing bacteria, about 23 genera.
The second largest group of sulfate-reducing bacteria is found among the Bacillota
The Bacillota (synonym Firmicutes) are a phylum of bacteria, most of which have Gram-positive cell wall structure. They have round cells, called cocci (singular coccus), or rod-like forms (bacillus). A few Bacillota, such as '' Megasphaera'', ...
, including the genera '' Desulfotomaculum'', '' Desulfosporomusa'', and '' Desulfosporosinus''.
In the Nitrospirota phylum we find sulfate-reducing '' Thermodesulfovibrio'' species.
Two more groups that include thermophilic
A thermophile is a type of extremophile that thrives at relatively high temperatures, between . Many thermophiles are archaea, though some of them are bacteria and fungi. Thermophilic eubacteria are suggested to have been among the earliest bact ...
sulfate-reducing bacteria are given their own phyla, the Thermodesulfobacteriota and '' Thermodesulfobium''.
There are also three known genera of sulfate-reducing archaea: '' Archaeoglobus'', '' Thermocladium'' and '' Caldivirga''. They are found in hydrothermal vents, oil deposits, and hot springs.
In July 2019, a scientific study of Kidd Mine in Canada discovered sulfate-reducing microorganisms living below the surface. The sulfate reducers discovered in Kidd Mine are lithotrophs, obtaining their energy by oxidizing minerals such as pyrite rather than organic compounds. Kidd Mine is also the site of the oldest known water on Earth.December 14, 2016, Maggie Romuld
See also
* Anaerobic respiration *Deep biosphere
The deep biosphere is the part of the biosphere that resides below the first few meters of the ocean's surface. It extends below the continental surface and below the sea surface, at temperatures that may reach beyond which is comparable to s ...
* Extremophile
* Microbial metabolism
* Microorganism
A microorganism, or microbe, is an organism of microscopic scale, microscopic size, which may exist in its unicellular organism, single-celled form or as a Colony (biology)#Microbial colonies, colony of cells. The possible existence of unseen ...
* Quinone-interacting membrane-bound oxidoreductase
* Sulfur cycle
References
External links
'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory
Garnet S. Lollar, Oliver Warr, Jon Telling, Magdalena R. Osburn & Barbara Sherwood Lollar, Received 15 Jan 2019, Accepted 01 Jul 2019, Published online: 18 Jul 2019.
Deep fracture fluids isolated in the crust since the Precambrian era
G. Holland, B. Sherwood Lollar, L. Li, G. Lacrampe-Couloume, G. F. Slater & C. J. Ballentine, Nature volume 497, pages 357–360 (16 May 2013)
Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks
by L. Li, B. A. Wing, T. H. Bui, J. M. McDermott, G. F. Slater, S. Wei, G. Lacrampe-Couloume & B. Sherwood Lollar October 27, 2016. Nature Communications volume 7, Article number: 13252 (2016.)
Earth's mysterious 'deep biosphere' may harbor millions of undiscovered species
By Brandon Specktor, Live Science, December 11, 2018, published online at nbcnews.com. {{DEFAULTSORT:Sulfate-Reducing Bacteria Bacteria Martinus Beijerinck Extremophiles Environmental microbiology Ecology Geomicrobiology Microbial growth and nutrition Thermodesulfobacteriota