Sulfate-reducing microorganism on:
[Wikipedia]
[Google]
[Amazon]
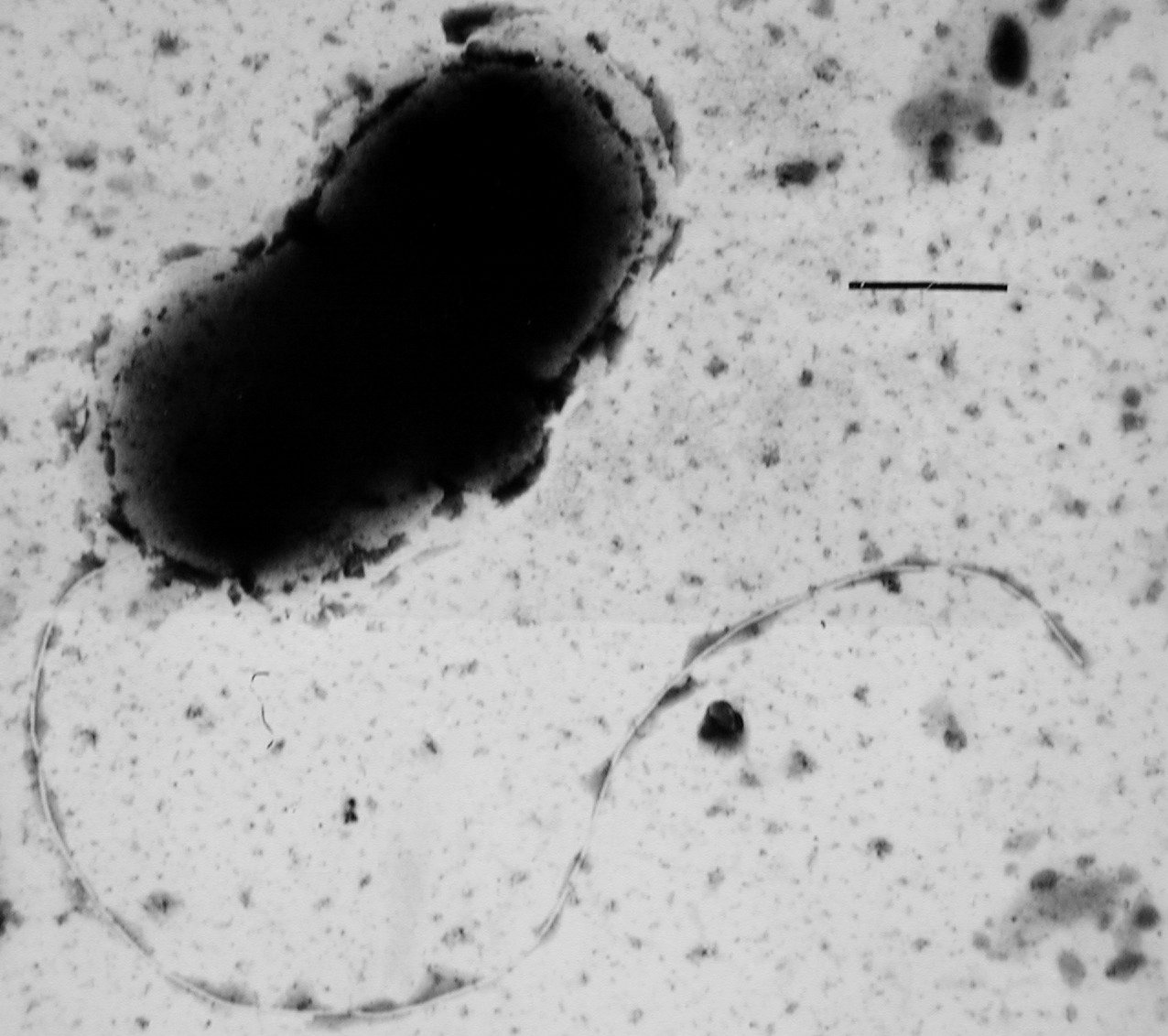 Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing
 The toxic
The toxic
 The enzyme dissimilatory (bi)sulfite reductase, ''dsrAB'' (EC 1.8.99.5), that catalyzes the last step of dissimilatory sulfate reduction, is the functional gene most used as a molecular marker to detect the presence of sulfate-reducing microorganisms.
The enzyme dissimilatory (bi)sulfite reductase, ''dsrAB'' (EC 1.8.99.5), that catalyzes the last step of dissimilatory sulfate reduction, is the functional gene most used as a molecular marker to detect the presence of sulfate-reducing microorganisms.
Oldest Water on Earth Found Deep Within the Canadian Shield
December 14, 2016, Maggie Romuld
'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory
Garnet S. Lollar, Oliver Warr, Jon Telling, Magdalena R. Osburn & Barbara Sherwood Lollar, Received 15 Jan 2019, Accepted 01 Jul 2019, Published online: 18 Jul 2019.
Deep fracture fluids isolated in the crust since the Precambrian era
G. Holland, B. Sherwood Lollar, L. Li, G. Lacrampe-Couloume, G. F. Slater & C. J. Ballentine, Nature volume 497, pages 357–360 (16 May 2013)
Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks
by L. Li, B. A. Wing, T. H. Bui, J. M. McDermott, G. F. Slater, S. Wei, G. Lacrampe-Couloume & B. Sherwood Lollar October 27, 2016. Nature Communications volume 7, Article number: 13252 (2016.)
Earth's mysterious 'deep biosphere' may harbor millions of undiscovered species
By Brandon Specktor, Live Science, December 11, 2018, published online at nbcnews.com. {{DEFAULTSORT:Sulfate-Reducing Bacteria Bacteria Martinus Beijerinck Extremophiles Environmental microbiology Ecology Geomicrobiology Microbial growth and nutrition
bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were am ...
(SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
In aerobic organisms undergoing ...
utilizing sulfate
The sulfate or sulphate ion is a polyatomic ion, polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salt (chemistry), ...
() as terminal electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mist ...
, reducing it to hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
(H2S). Therefore, these sulfidogenic microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s "breathe" sulfate rather than molecular oxygen
There are several known allotropy, allotropes of oxygen. The most familiar is oxygen, molecular oxygen (O2), present at significant levels in Atmosphere of Earth, Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the hig ...
(O2), which is the terminal electron acceptor reduced to water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
(H2O) in aerobic respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
.
Most sulfate-reducing microorganisms can also reduce some other oxidized inorganic sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
compounds, such as sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are wide ...
(), dithionite
The dithionite is the oxyanion with the formula 2O4sup>2−. It is commonly encountered as the salt sodium dithionite. For historical reasons, it is sometimes called hydrosulfite, but it contains no hydrogen and is not a sulfite. The diani ...
(), thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
(), trithionate
Trithionate is an oxyanion of sulfur with the chemical formula . It is the conjugate base of trithionic acid. Dilute sodium hydroxide hydrolyzes as follows, yielding sodium thiosulfate and sodium trithionate:
2 + 6 NaOH + 9 → + 2 + 8
Ce ...
(), tetrathionate
The tetrathionate anion, , is a sulfur oxoanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5. Alternatively, the compound can be viewe ...
(), elemental sulfur (S8), and polysulfide
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. Among the inorganic polysulfides, there are ones which contain anions, which have the general formu ...
s (). Depending on the context, "sulfate-reducing microorganisms" can be used in a broader sense (including all species that can reduce any of these sulfur compounds) or in a narrower sense (including only species that reduce sulfate, and excluding strict thiosulfate and sulfur reducers, for example).
Sulfate-reducing microorganisms can be traced back to 3.5 billion years ago and are considered to be among the oldest forms of microbes, having contributed to the sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element ( CHNOPS), being a cons ...
soon after life emerged on Earth.
Many organisms reduce small amounts of sulfates in order to synthesize sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
-containing cell components; this is known as ''assimilatory sulfate reduction''. By contrast, the sulfate-reducing microorganisms considered here reduce sulfate in large amounts to obtain energy and expel the resulting sulfide as waste; this is known as dissimilatory sulfate reduction
Dissimilatory sulfate reduction is a form of anaerobic respiration that uses sulfate as the terminal electron acceptor to produce hydrogen sulfide. This metabolism is found in some types of bacteria and archaea which are often termed sulfate-redu ...
. They use sulfate as the terminal electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mist ...
of their electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couple ...
. Most of them are anaerobes
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenate ...
; however, there are examples of sulfate-reducing microorganisms that are tolerant of oxygen, and some of them can even perform aerobic respiration.
No growth is observed when oxygen is used as the electron acceptor.
"
In addition, there are sulfate-reducing microorganisms that can also reduce other electron acceptors, such as fumarate
Fumaric acid is an organic compound with the formula HO2CCH=CHCO2H. A white solid, fumaric acid occurs widely in nature. It has a fruit-like taste and has been used as a food additive. Its E number is E297.
The salts and esters are known as f ...
, nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble ...
(), nitrite
The nitrite ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also re ...
(), ferric
In chemistry, iron(III) refers to the element iron in its +3 oxidation state. In ionic compounds (salts), such an atom may occur as a separate cation (positive ion) denoted by Fe3+.
The adjective ferric or the prefix ferri- is often used to s ...
iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
(Fe3+), and dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds ...
(DMSO).
In terms of electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent che ...
, this group contains both organotroph
An organotroph is an organism that obtains hydrogen or electrons from organic substrates. This term is used in microbiology to classify and describe organisms based on how they obtain electrons for their respiration processes. Some organotrophs su ...
s and lithotroph
Lithotrophs are a diverse group of organisms using an inorganic substrate (usually of mineral origin) to obtain reducing equivalents for use in biosynthesis (e.g., carbon dioxide fixation) or energy conservation (i.e., ATP production) via aerob ...
s. The organotrophs oxidize
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s, such as carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or ...
s, organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are ...
s (such as formate
Formate ( IUPAC name: methanoate) is the conjugate base of formic acid. Formate is an anion () or its derivatives such as ester of formic acid. The salts and esters are generally colorless.Werner Reutemann and Heinz Kieczka "Formic Acid" in ...
, lactate
Lactate may refer to:
* Lactation, the secretion of milk from the mammary glands
* Lactate, the conjugate base of lactic acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with ...
, acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
, propionate
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a liqu ...
, and butyrate
The conjugate acids are in :Carboxylic acids.
{{Commons category, Carboxylate ions, Carboxylate anions
Carbon compounds
Oxyanions ...
), alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s ( methanol and ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
), aliphatic hydrocarbons
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or ...
(including methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
), and aromatic hydrocarbons (benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
, ethylbenzene
Ethylbenzene is an organic compound with the formula . It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as an reaction intermedia ...
, and xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are sub ...
). The lithotrophs oxidize molecular hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, ...
(H2), for which they compete with methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens ar ...
s and acetogen
An acetogen is a microorganism that generates acetate (CH3COO−) as an end product of anaerobic respiration or fermentation. However, this term is usually employed in a narrower sense only to those bacteria and archaea that perform anaerobic res ...
s in anaerobic conditions. Some sulfate-reducing microorganisms can directly use metallic iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
(Fe0, also known as zerovalent iron
Zerovalent iron (ZVI) is jargon that describes forms of iron metal used for Groundwater remediation. ZVI serves as a reducing agent.Gillham, Robert, John Vogan, Lai Gui, Michael Duchene, and Jennifer Son. "Iron Barrier Walls for Chlorinated Solven ...
, or ZVI) as electron donor, oxidizing it to ferrous iron (Fe2+).
Ecological importance and markers
Sulfate occurs widely in seawater, sediment, and water rich in decaying organic material. Sulfate is also found in more extreme environments such as hydrothermal vents,acid mine drainage
Acid mine drainage, acid and metalliferous drainage (AMD), or acid rock drainage (ARD) is the outflow of acidic water from metal mines or coal mines.
Acid rock drainage occurs naturally within some environments as part of the rock weathering ...
sites, oil fields, and the deep subsurface, including the world's oldest isolated ground water. Sulfate-reducing microorganisms are common in anaerobic
Anaerobic means "living, active, occurring, or existing in the absence of free oxygen", as opposed to aerobic which means "living, active, or occurring only in the presence of oxygen." Anaerobic may also refer to:
*Adhesive#Anaerobic, Anaerobic ad ...
environments where they aid in the degradation of organic materials. In these anaerobic environments, fermenting bacteria extract energy from large organic molecules; the resulting smaller compounds such as organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are ...
s and alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s are further oxidized by acetogen
An acetogen is a microorganism that generates acetate (CH3COO−) as an end product of anaerobic respiration or fermentation. However, this term is usually employed in a narrower sense only to those bacteria and archaea that perform anaerobic res ...
s and methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens ar ...
s and the competing sulfate-reducing microorganisms.
 The toxic
The toxic hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
is a waste product of sulfate-reducing microorganisms; its rotten egg odor is often a marker for the presence of sulfate-reducing microorganisms in nature.
Sulfate-reducing microorganisms are responsible for the sulfurous odors of salt marshes and mud flats. Much of the hydrogen sulfide will react with metal ions in the water to produce metal sulfides. These metal sulfides, such as ferrous sulfide
Iron(II) sulfide or ferrous sulfide (Br.E. sulphide) is one of a family chemical compounds and minerals with the approximate formula . Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids.
Preparatio ...
(FeS), are insoluble and often black or brown, leading to the dark color of sludge.
During the Permian–Triassic extinction event
The Permian–Triassic (P–T, P–Tr) extinction event, also known as the Latest Permian extinction event, the End-Permian Extinction and colloquially as the Great Dying, formed the boundary between the Permian and Triassic geologic periods, a ...
(250 million years ago) a severe anoxic event
Oceanic anoxic events or anoxic events (anoxia conditions) describe periods wherein large expanses of Earth's oceans were depleted of dissolved oxygen (O2), creating toxic, euxinic (anoxic and sulfidic) waters. Although anoxic events have not ...
seems to have occurred where these forms of bacteria became the dominant force in oceanic ecosystems, producing copious amounts of hydrogen sulfide.
Sulfate-reducing bacteria also generate neurotoxic methylmercury
Methylmercury (sometimes methyl mercury) is an organometallic cation with the formula . It is the simplest organomercury compound. Methylmercury is extremely toxic, and its derivatives are the major source of organic mercury for humans. It is ...
as a byproduct of their metabolism, through methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These t ...
of inorganic mercury present in their surroundings. They are known to be the dominant source of this bioaccumulative
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance at a rate faster than that at which the substance is lost or eliminated ...
form of mercury in aquatic systems.
Uses
Some sulfate-reducing microorganisms can reducehydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, and they have been used to clean up contaminated soils. Their use has also been proposed for other kinds of contaminations.
Sulfate-reducing microorganisms are considered a possible way to deal with acid mine waters that are produced by other microorganisms.
Problems caused by sulfate-reducing microorganisms
In engineering, sulfate-reducing microorganisms can create problems when metal structures are exposed to sulfate-containing water: Interaction of water and metal creates a layer of molecular hydrogen on the metal surface; sulfate-reducing microorganisms then oxidize the hydrogen while creating hydrogen sulfide, which contributes tocorrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
.
Hydrogen sulfide from sulfate-reducing microorganisms also plays a role in the biogenic sulfide corrosion Biogenic sulfide corrosion is a bacterially mediated process of forming hydrogen sulfide gas and the subsequent conversion to sulfuric acid that attacks concrete and steel within wastewater environments. The hydrogen sulfide gas is biochemically ...
of concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most ...
. It also occurs in sour crude oil Sour crude oil is crude oil containing a high amount of the impurity sulfur. It is common to find crude oil containing some impurities. When the total sulfur level in the oil is more than 0.5% (by weight), the oil is called "sour".
The impurities ...
.
Some sulfate-reducing microorganisms play a role in the anaerobic oxidation of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
:
:CH4 + → + HS− + H2O
An important fraction of the methane formed by methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in hypoxic conditions. They are prokaryotic and belong to the domain Archaea. All known methanogens are members of the archaeal phylum Euryarchaeota. Methanogens ar ...
s below the seabed is oxidized by sulfate-reducing microorganisms in the transition zone separating the methanogenesis from the sulfate reduction activity in the sediments. This process is also considered a major sink for sulfate in marine sediments.
In hydraulic fracturing
Fracking (also known as hydraulic fracturing, hydrofracturing, or hydrofracking) is a well stimulation technique involving the fracturing of bedrock formations by a pressurized liquid. The process involves the high-pressure injection of "frac ...
, fluids are used to frack shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4) and tiny fragments (silt-sized particles) of other minerals, especia ...
formation
Formation may refer to:
Linguistics
* Back-formation, the process of creating a new lexeme by removing or affixes
* Word formation, the creation of a new word by adding affixes
Mathematics and science
* Cave formation or speleothem, a secondary ...
s to recover methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
(shale gas
Shale gas is an unconventional natural gas that is found trapped within shale formations. Since the 1990s a combination of horizontal drilling and hydraulic fracturing has made large volumes of shale gas more economical to produce, and some ...
) and hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
. Biocide
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a slig ...
compounds are often added to water to inhibit the microbial activity of sulfate-reducing microorganisms, in order to but not limited to, avoid anaerobic methane oxidation and the generation of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
, ultimately resulting in minimizing potential production loss.
Biochemistry
Before sulfate can be used as an electron acceptor, it must be activated. This is done by the enzymeATP-sulfurylase
In enzymology, a sulfate adenylyltransferase () is an enzyme that catalyzes the chemical reaction
:ATP + sulfate \rightleftharpoonspyrophosphate + adenylyl sulfate
Thus, the two substrates of this enzyme are ATP and sulfate, whereas its two p ...
, which uses ATP
ATP may refer to:
Companies and organizations
* Association of Tennis Professionals, men's professional tennis governing body
* American Technical Publishers, employee-owned publishing company
* ', a Danish pension
* Armenia Tree Project, non ...
and sulfate to create adenosine 5′-phosphosulfate (APS). APS is subsequently reduced to sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are wide ...
and AMP #REDIRECT Amp
{{Redirect category shell, {{R from other capitalisation{{R from ambiguous page ...
. Sulfite is then further reduced to sulfide, while AMP is turned into ADP
Adp or ADP may refer to:
Aviation
* Aéroports de Paris, airport authority for the Parisian region in France
* Aeropuertos del Perú, airport operator for airports in northern Peru
* SLAF Anuradhapura, an airport in Sri Lanka
* Ampara Air ...
using another molecule of ATP. The overall process, thus, involves an investment of two molecules of the energy carrier ATP, which must to be regained from the reduction.
Phylogeny
The sulfate-reducing microorganisms have been treated as a phenotypic group, together with the othersulfur-reducing bacteria
Sulfur-reducing bacteria are microorganisms able to reduce elemental sulfur (S0) to hydrogen sulfide (H2S). These microbes use inorganic sulfur compounds as electron acceptors to sustain several activities such as respiration, conserving energy an ...
, for identification purposes. They are found in several different phylogenetic lines. As of 2009, 60 genera containing 220 species of sulfate-reducing bacteria are known.
Among the Thermodesulfobacteriota
The Thermodesulfobacteriota are a phylum of thermophilic sulfate-reducing bacteria.
A pathogenic intracellular thermodesulfobacteriote has recently been identified.
Phylogeny
The phylogeny is based on phylogenomic analysis:
See also
* ...
the orders of sulfate-reducing bacteria include Desulfobacterales, Desulfovibrionales, and Syntrophobacterales
The Syntrophobacterales are an order of Thermodesulfobacteriota. All genera are strictly anaerobic.Garrity, George M.; Brenner, Don J.; Krieg, Noel R.; Staley, James T. (eds.) (2005). Bergey's Manual of Systematic Bacteriology, Volume Two: The P ...
. This accounts for the largest group of sulfate-reducing bacteria, about 23 genera.
The second largest group of sulfate-reducing bacteria is found among the Bacillota
The Bacillota (synonym Firmicutes) are a phylum of bacteria, most of which have gram-positive cell wall structure. The renaming of phyla such as Firmicutes in 2021 remains controversial among microbiologists, many of whom continue to use the ea ...
, including the genera '' Desulfotomaculum'', '' Desulfosporomusa'', and '' Desulfosporosinus''.
In the Nitrospirota
Nitrospirota is a phylum of bacteria. It includes multiple genera, such as '' Nitrospira'', the largest. The first member of this phylum, '' Nitrospira marina'', was discovered in 1985. The second member, '' Nitrospira moscoviensis'', was discove ...
phylum we find sulfate-reducing '' Thermodesulfovibrio'' species.
Two more groups that include thermophilic
A thermophile is an organism—a type of extremophile—that thrives at relatively high temperatures, between . Many thermophiles are archaea, though they can be bacteria or fungi. Thermophilic eubacteria are suggested to have been among the ear ...
sulfate-reducing bacteria are given their own phyla, the Thermodesulfobacteriota
The Thermodesulfobacteriota are a phylum of thermophilic sulfate-reducing bacteria.
A pathogenic intracellular thermodesulfobacteriote has recently been identified.
Phylogeny
The phylogeny is based on phylogenomic analysis:
See also
* ...
and '' Thermodesulfobium''.
There are also three known genera of sulfate-reducing archaea: ''Archaeoglobus
''Archaeoglobus'' is a genus of the phylum Euryarchaeota. ''Archaeoglobus'' can be found in high-temperature oil fields where they may contribute to oil field souring.
Metabolism
''Archaeoglobus'' grow anaerobically at extremely high temperat ...
'', ''Thermocladium
In taxonomy, ''Thermocladium'' is a genus of the Thermoproteaceae.See the NCBI
The National Center for Biotechnology Information (NCBI) is part of the United States National Library of Medicine (NLM), a branch of the National Institutes of H ...
'' and '' Caldivirga''. They are found in hydrothermal vents, oil deposits, and hot springs.
In July 2019, a scientific study of Kidd Mine
Kidd Mine or Kidd Creek Mine is an underground base metal mine north of Timmins, Ontario, Canada. It is owned and operated by Swiss multinational Glencore Inc. The mine was discovered in 1963 by Texas Gulf Sulfur Company. In 1981 it was sold to ...
in Canada discovered sulfate-reducing microorganisms living below the surface. The sulfate reducers discovered in Kidd Mine are lithotrophs, obtaining their energy by oxidizing minerals such as pyrite rather than organic compounds. Kidd Mine is also the site of the oldest known water on Earth.December 14, 2016, Maggie Romuld
See also
*Anaerobic respiration
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.
In aerobic organisms undergoing ...
* Deep biosphere
The deep biosphere is the part of the biosphere that resides below the first few meters of the surface. It extends down at least 5 kilometers below the continental surface and 10.5 kilometers below the sea surface, at temperatures that ...
* Extremophile
An extremophile (from Latin ' meaning "extreme" and Greek ' () meaning "love") is an organism that is able to live (or in some cases thrive) in extreme environments, i.e. environments that make survival challenging such as due to extreme tempe ...
* Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other ...
* Microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
* Quinone-interacting membrane-bound oxidoreductase
* Sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element ( CHNOPS), being a cons ...
References
External links
'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory
Garnet S. Lollar, Oliver Warr, Jon Telling, Magdalena R. Osburn & Barbara Sherwood Lollar, Received 15 Jan 2019, Accepted 01 Jul 2019, Published online: 18 Jul 2019.
Deep fracture fluids isolated in the crust since the Precambrian era
G. Holland, B. Sherwood Lollar, L. Li, G. Lacrampe-Couloume, G. F. Slater & C. J. Ballentine, Nature volume 497, pages 357–360 (16 May 2013)
Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks
by L. Li, B. A. Wing, T. H. Bui, J. M. McDermott, G. F. Slater, S. Wei, G. Lacrampe-Couloume & B. Sherwood Lollar October 27, 2016. Nature Communications volume 7, Article number: 13252 (2016.)
Earth's mysterious 'deep biosphere' may harbor millions of undiscovered species
By Brandon Specktor, Live Science, December 11, 2018, published online at nbcnews.com. {{DEFAULTSORT:Sulfate-Reducing Bacteria Bacteria Martinus Beijerinck Extremophiles Environmental microbiology Ecology Geomicrobiology Microbial growth and nutrition