Stille coupling on:
[Wikipedia]
[Google]
[Amazon]
The Stille reaction is a
Review
Farina, V.; Krishnamurthy, V.; Scott, W. J. ''Org. React.'' 1998, ''50'', 1–652.
Review
: :*: Allyl, alkenyl, aryl, benzyl, acyl :*: halides (Cl, Br, I), pseudohalides (OTf, OPO(OR)2), OAc The R1 group attached to the trialkyltin is normally sp2-hybridized, including
Article
Stille, J. K.; Echavarren, A. M.; Williams, R. M.; Hendrix, J. A. ''
Article
Several reviews have been published.Kurti, L.; Czako, B. ''Strategic Applications of Named Reactions in Organic Synthesis''; Elsevier: Burlington, 2005.Mitchell, T. N. ''J. Organomet. Chem.'', 1986, ''304'', 1–16.Mitchell, T. N. ''Synthesis'', 1992, 803–815. ()Farina, V. ''Pure Appl. Chem.'', 1996, ''68'', 73–78. ().Farina, V.; Krishnamurthy, V.; Scott, W. J. ''The Stille Reaction''; Wiley: Online, 2004. ().Espinet, P.; Echavarren, A. M. ''Angew. Chem. Int. Ed.'', 2004, ''43'', 4704–4734.()Pattenden, G.; Sinclair, D. J. ''J.Organomet. Chem.'', 2002, 653, 261–268.Kosugi, M.; Fugami, K. ''J. Organomet. Chem.'', 2002, 19, 10–16.Pierre Genet, J.; Savignac, M. ''J. Organomet. Chem.'', 1999, 576, 305–317.Cordova, C.; Bartolomé, C.; Martínez-Ilarduya, J.M..; Espinet, P. ''ACS Catal''., 2015, ''5'', 3040–3053.().
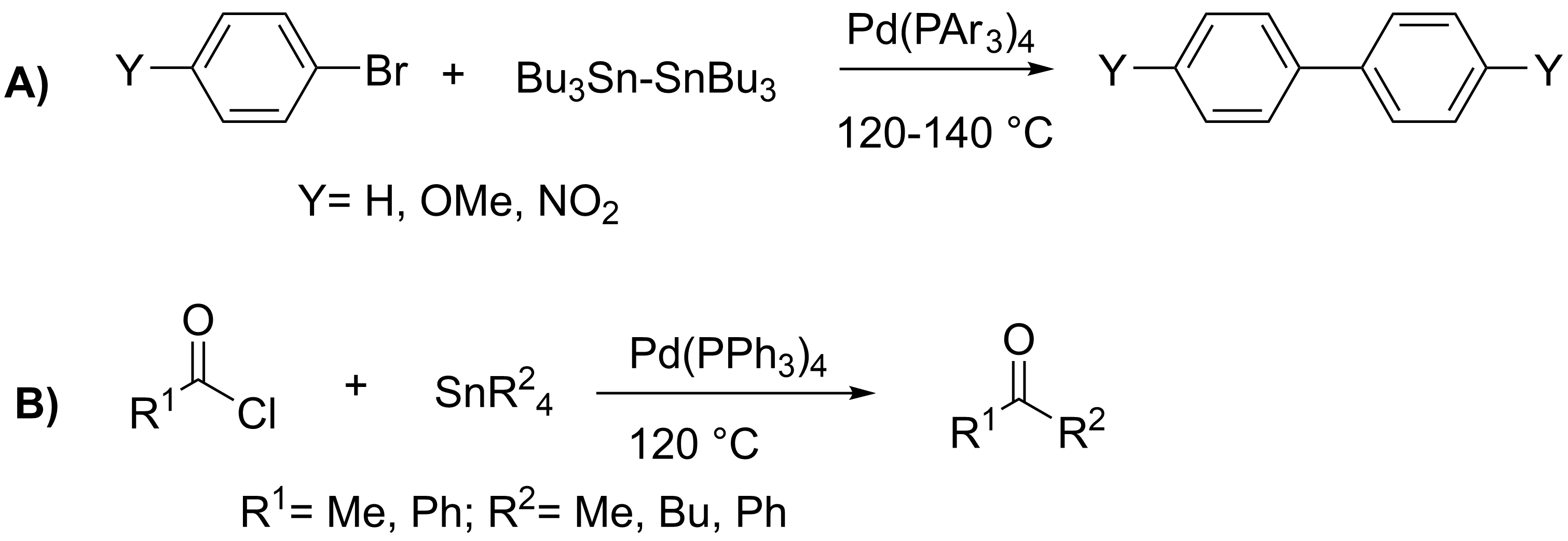 In 1977, Migita published further work on the coupling of
In 1977, Migita published further work on the coupling of 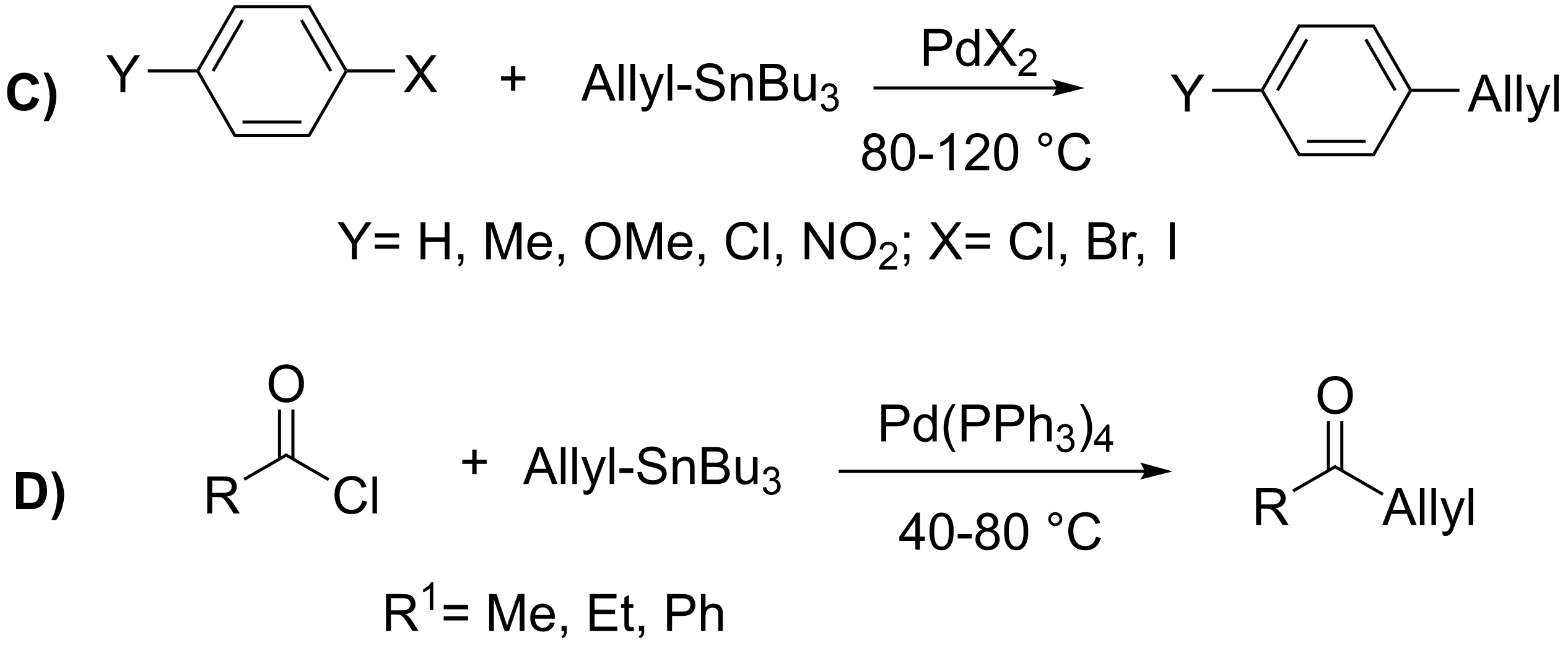 John Kenneth Stille subsequently reported the coupling of a variety of alkyl tin reagents in 1978 with numerous aryl and acyl halides under mild reaction conditions with much better yields (76%–99%).Milstein, D.; Stille, J. K. ''
John Kenneth Stille subsequently reported the coupling of a variety of alkyl tin reagents in 1978 with numerous aryl and acyl halides under mild reaction conditions with much better yields (76%–99%).Milstein, D.; Stille, J. K. '' By the mid-1980s, over 65 papers on the topic of coupling reactions involving tin had been published, continuing to explore the substrate scope of this reaction. While initial research in the field focused on the coupling of alkyl groups, most future work involved the much more synthetically useful coupling of
By the mid-1980s, over 65 papers on the topic of coupling reactions involving tin had been published, continuing to explore the substrate scope of this reaction. While initial research in the field focused on the coupling of alkyl groups, most future work involved the much more synthetically useful coupling of
 However, the detailed mechanism of the Stille coupling is extremely complex and can occur via numerous reaction pathways. Like other palladium-catalyzed coupling reactions, the active palladium catalyst is believed to be a 14-electron Pd(0) complex, which can be generated in a variety of ways. Use of an 18- or 16- electron Pd(0) source , can undergo
However, the detailed mechanism of the Stille coupling is extremely complex and can occur via numerous reaction pathways. Like other palladium-catalyzed coupling reactions, the active palladium catalyst is believed to be a 14-electron Pd(0) complex, which can be generated in a variety of ways. Use of an 18- or 16- electron Pd(0) source , can undergo
 There are multiple reasons why
There are multiple reasons why
 A less common pathway for transmetalation is through a dissociative or solvent assisted mechanism. Here, a ligand from the tetravalent palladium species dissociates, and a coordinating solvent can add onto the palladium. When the
A less common pathway for transmetalation is through a dissociative or solvent assisted mechanism. Here, a ligand from the tetravalent palladium species dissociates, and a coordinating solvent can add onto the palladium. When the
 The previous process, however, is sometimes slow and can be greatly accelerated by dissociation of a ligand to yield a 14-electron T shaped intermediate. This intermediate can then rearrange to form a Y-shaped adduct, which can undergo faster reductive elimination.
The previous process, however, is sometimes slow and can be greatly accelerated by dissociation of a ligand to yield a 14-electron T shaped intermediate. This intermediate can then rearrange to form a Y-shaped adduct, which can undergo faster reductive elimination.
 Finally, an extra ligand can associate to the palladium to form an 18-electron trigonal bipyramidal structure, with R1 and R2 cis to each other in equatorial positions. The geometry of this intermediate makes it similar to the Y-shaped above.
Finally, an extra ligand can associate to the palladium to form an 18-electron trigonal bipyramidal structure, with R1 and R2 cis to each other in equatorial positions. The geometry of this intermediate makes it similar to the Y-shaped above.
 The presence of bulky ligands can also increase the rate of elimination. Ligands such as phosphines with large bite angles cause steric repulsion between L and R1 and R2, resulting in the angle between L and the R groups to increase and the angle between R1 and R2 to hence decrease, allowing for quicker reductive elimination.
The presence of bulky ligands can also increase the rate of elimination. Ligands such as phosphines with large bite angles cause steric repulsion between L and R1 and R2, resulting in the angle between L and the R groups to increase and the angle between R1 and R2 to hence decrease, allowing for quicker reductive elimination.

 As the organic tin compound, a trimethylstannyl or tributylstannyl compound is normally used. Although trimethylstannyl compounds show higher reactivity compared with tributylstannyl compounds and have much simpler 1H-NMR spectra, the toxicity of the former is much larger.
Optimizing which ligands are best at carrying out the reaction with high yield and turnover rate can be difficult. This is because the oxidative addition requires an electron rich metal, hence favoring electron donating ligands. However, an electron deficient metal is more favorable for the transmetalation and reductive elimination steps, making electron withdrawing ligands the best here. Therefore, the optimal ligand set heavily depends on the individual substrates and conditions used. These can change the rate determining step, as well as the mechanism for the transmetalation step.Farina, V.; ''Journal of the American Chemical Society'', 1991, ''113'', 9585–9595. ().
Normally, ligands of intermediate donicity, such as phosphines, are utilized. Rate enhancements can be seen when moderately electron-poor ligands, such as tri-2-furylphosphine or triphenylarsenine are used. Likewise, ligands of high donor number can slow down or inhibit coupling reactions.
These observations imply that normally, the rate-determining step for the Stille reaction is transmetalation.
As the organic tin compound, a trimethylstannyl or tributylstannyl compound is normally used. Although trimethylstannyl compounds show higher reactivity compared with tributylstannyl compounds and have much simpler 1H-NMR spectra, the toxicity of the former is much larger.
Optimizing which ligands are best at carrying out the reaction with high yield and turnover rate can be difficult. This is because the oxidative addition requires an electron rich metal, hence favoring electron donating ligands. However, an electron deficient metal is more favorable for the transmetalation and reductive elimination steps, making electron withdrawing ligands the best here. Therefore, the optimal ligand set heavily depends on the individual substrates and conditions used. These can change the rate determining step, as well as the mechanism for the transmetalation step.Farina, V.; ''Journal of the American Chemical Society'', 1991, ''113'', 9585–9595. ().
Normally, ligands of intermediate donicity, such as phosphines, are utilized. Rate enhancements can be seen when moderately electron-poor ligands, such as tri-2-furylphosphine or triphenylarsenine are used. Likewise, ligands of high donor number can slow down or inhibit coupling reactions.
These observations imply that normally, the rate-determining step for the Stille reaction is transmetalation.
Article
 It has also been found that at temperatures as low as 50 °C,
It has also been found that at temperatures as low as 50 °C,  Finally, a rather rare and exotic
Finally, a rather rare and exotic  Numerous other side reactions can occur, and these include E/Z isomerization, which can potentially be a problem when an alkenylstannane is utilized. The mechanism of this transformation is currently unknown. Normally, organostannanes are quite stable to
Numerous other side reactions can occur, and these include E/Z isomerization, which can potentially be a problem when an alkenylstannane is utilized. The mechanism of this transformation is currently unknown. Normally, organostannanes are quite stable to
 Normally, the
Normally, the  Another class of common electrophiles are aryl and
Another class of common electrophiles are aryl and  Below is an example of the use of Stille coupling to build complexity on heterocycles of
Below is an example of the use of Stille coupling to build complexity on heterocycles of  Aryl triflates and
Aryl triflates and 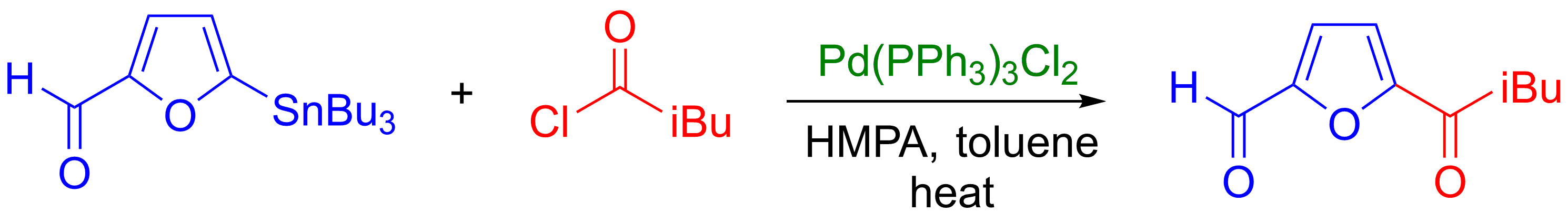

 Arylstannane reagents are also common and both electron donating and electron withdrawing groups actually increase the rate of the transmetalation. This again implies that two mechanisms of transmetalation can occur. The only limitation to these reagents are substituents at the ortho-position as small as methyl groups can decrease the rate of reaction. A wide variety of heterocycles (see Electrophile section) can also be used as coupling partners (see example with a thiazole ring below).Bailey, T. R. ''Tetrahedron Lett''., 1986, ''27'', 4407. ().
Arylstannane reagents are also common and both electron donating and electron withdrawing groups actually increase the rate of the transmetalation. This again implies that two mechanisms of transmetalation can occur. The only limitation to these reagents are substituents at the ortho-position as small as methyl groups can decrease the rate of reaction. A wide variety of heterocycles (see Electrophile section) can also be used as coupling partners (see example with a thiazole ring below).Bailey, T. R. ''Tetrahedron Lett''., 1986, ''27'', 4407. ().

 Alkynylstannanes, the most reactive of stannanes, have also been used in Stille couplings. They are not usually needed as terminal alkynes can couple directly to palladium catalysts through their C-H bond via
Alkynylstannanes, the most reactive of stannanes, have also been used in Stille couplings. They are not usually needed as terminal alkynes can couple directly to palladium catalysts through their C-H bond via
 Panek's 32 step enantioselective
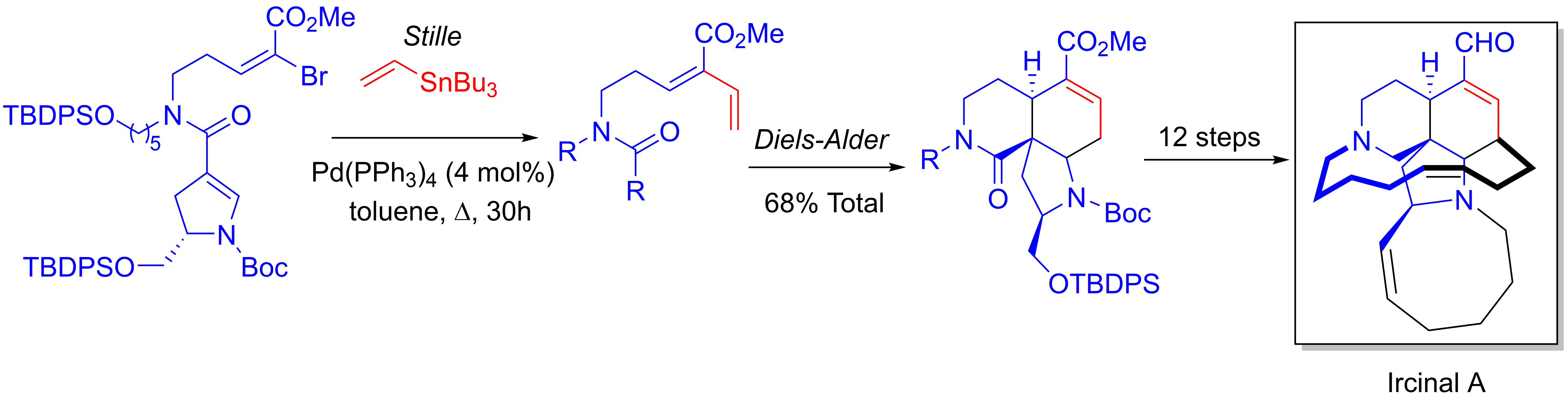
Panek's 32 step enantioselective  Stephen F. Martin and coworkers' 21 step enantioselective total synthesis of the manzamine antitumor alkaloid Ircinal A makes use of a tandem one-pot Stille/Diels-Alder reaction. An alkene group is added to vinyl bromide, followed by an ''in situ'' Diels-Alder cycloaddition between the added alkene and the alkene in the pyrrolidine ring.Martin, S. F.; Humphrey, J. M.; Ali, A.; Hillier, M. C. ''Journal of the American Chemical Society'', 1999, ''121'', 866–867. ()
Stephen F. Martin and coworkers' 21 step enantioselective total synthesis of the manzamine antitumor alkaloid Ircinal A makes use of a tandem one-pot Stille/Diels-Alder reaction. An alkene group is added to vinyl bromide, followed by an ''in situ'' Diels-Alder cycloaddition between the added alkene and the alkene in the pyrrolidine ring.Martin, S. F.; Humphrey, J. M.; Ali, A.; Hillier, M. C. ''Journal of the American Chemical Society'', 1999, ''121'', 866–867. ()
 Numerous other total syntheses utilize the Stille reaction, including those of oxazolomycin,Kende, A. S.; Kawamura, K.; DeVita, R. J. ''Journal of the American Chemical Society'', 1990, ''112'' 4070–4072. (). lankacidin C,Kende, A. S., Koch, K.; Dorey, G.; Kaldor, I.; Liu, K. ''Journal of the American Chemical Society'', 1993, ''115'', 9842–9843. (). onamide A,Hong, C. Y, Kishi, Y. ''Journal of the American Chemical Society'', 1991, ''113'', 9693–9694. (). calyculin A,Tanimoto, N.; Gerritz, S. W.; Sawabe, A.; Noda, T.; Filla, S. A.; Masamune, S. ''Angew. Chem. Int. Ed.'', 2003, ''33'', 673–675. (). lepicidin A,Evans, D. A.; Black, W. C. ''Journal of the American Chemical Society'', 1993, ''115'', 4497–4513. (). ripostatin A,Tang, W.; Prusov, E. V. ''Org. Lett.'', 2012, ''14'' 4690–4693. (). and lucilactaene.Coleman, R. S.; Walczak, M. C.; Campbell, E. L. ''Journal of the American Chemical Society'', 2005, ''127'', 16036-16039. (). The image below displays the final
Numerous other total syntheses utilize the Stille reaction, including those of oxazolomycin,Kende, A. S.; Kawamura, K.; DeVita, R. J. ''Journal of the American Chemical Society'', 1990, ''112'' 4070–4072. (). lankacidin C,Kende, A. S., Koch, K.; Dorey, G.; Kaldor, I.; Liu, K. ''Journal of the American Chemical Society'', 1993, ''115'', 9842–9843. (). onamide A,Hong, C. Y, Kishi, Y. ''Journal of the American Chemical Society'', 1991, ''113'', 9693–9694. (). calyculin A,Tanimoto, N.; Gerritz, S. W.; Sawabe, A.; Noda, T.; Filla, S. A.; Masamune, S. ''Angew. Chem. Int. Ed.'', 2003, ''33'', 673–675. (). lepicidin A,Evans, D. A.; Black, W. C. ''Journal of the American Chemical Society'', 1993, ''115'', 4497–4513. (). ripostatin A,Tang, W.; Prusov, E. V. ''Org. Lett.'', 2012, ''14'' 4690–4693. (). and lucilactaene.Coleman, R. S.; Walczak, M. C.; Campbell, E. L. ''Journal of the American Chemical Society'', 2005, ''127'', 16036-16039. (). The image below displays the final 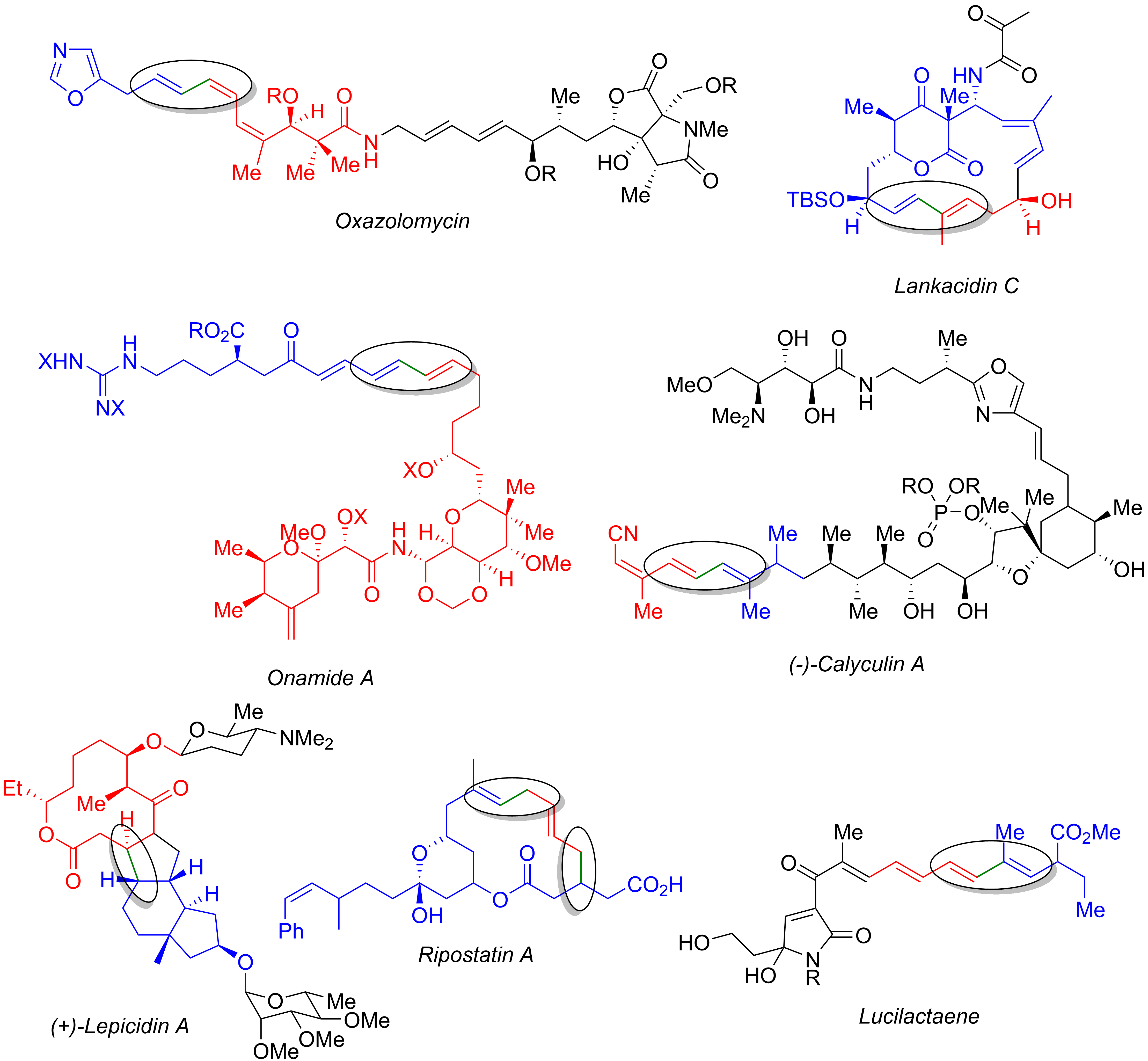
Article
In the realm of
 Larry Overman and coworkers make use of the Stille-carbonylative cross-coupling in their 20-step enantioselective
Larry Overman and coworkers make use of the Stille-carbonylative cross-coupling in their 20-step enantioselective 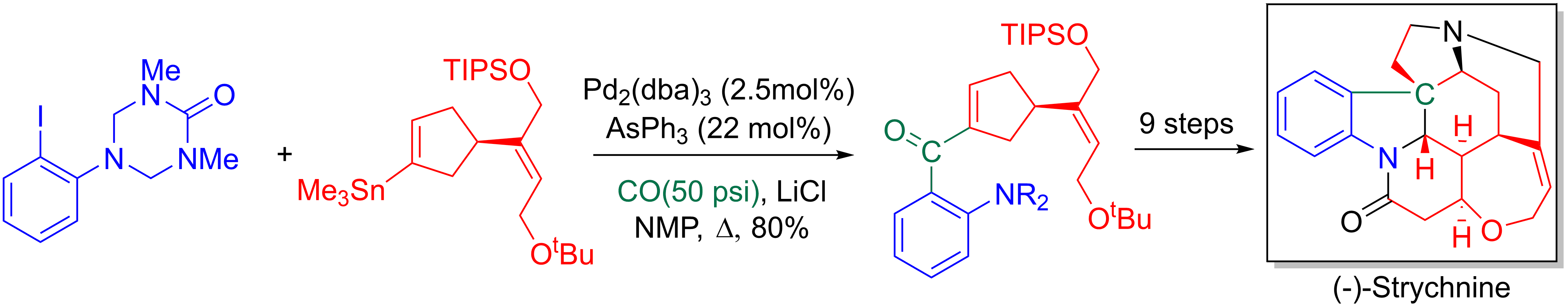 Giorgio Ortar et al. explored how the Stille-carbonylative cross-coupling could be used to synthesize
Giorgio Ortar et al. explored how the Stille-carbonylative cross-coupling could be used to synthesize  Louis Hegedus' 16-step
Louis Hegedus' 16-step 
 Jie Jack Lie et al. made use of the Stille-Kelly coupling in their synthesis of a variety of benzo ,5uropyridines ring systems. They invoke a three-step process, involving a Buchwald-Hartwig amination, another palladium-catalyzed coupling reaction, followed by an intramolecular Stille-Kelly coupling. Note that the aryl-iodide bond will oxidatively add to the
Jie Jack Lie et al. made use of the Stille-Kelly coupling in their synthesis of a variety of benzo ,5uropyridines ring systems. They invoke a three-step process, involving a Buchwald-Hartwig amination, another palladium-catalyzed coupling reaction, followed by an intramolecular Stille-Kelly coupling. Note that the aryl-iodide bond will oxidatively add to the 
Stille reaction handout
from the Myers group.
Stille reaction
at organic-chemistry.org Carbon-carbon bond forming reactions Palladium Name reactions
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
widely used in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered ...
(also known as organostannanes). A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.Hartwig, J. F. ''Organotransition Metal Chemistry, from Bonding to Catalysis''; University Science Books: New York, 2010. Stille, J. K. '' Angew. Chem. Int. Ed. Engl.'' 1986, ''25'', 508–524.Review
Farina, V.; Krishnamurthy, V.; Scott, W. J. ''Org. React.'' 1998, ''50'', 1–652.
Review
: :*: Allyl, alkenyl, aryl, benzyl, acyl :*: halides (Cl, Br, I), pseudohalides (OTf, OPO(OR)2), OAc The R1 group attached to the trialkyltin is normally sp2-hybridized, including
vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
, and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
groups.
These organostannanes are also stable to both air and moisture, and many of these reagents either are commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
, such as Cl, Br, or I, yet pseudohalides such as triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opp ...
s and sulfonate
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of ...
s and phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
s can also be used.Scott, W. J.; Crisp, G. T.; Stille, J. K. ''Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
'', Coll. Vol. 8, p. 97 (1993); Vol. 68, p. 116 (1990).Article
Stille, J. K.; Echavarren, A. M.; Williams, R. M.; Hendrix, J. A. ''
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
'', Coll. Vol. 9, p. 553 (1998); Vol. 71, p. 97 (1993).Article
Several reviews have been published.Kurti, L.; Czako, B. ''Strategic Applications of Named Reactions in Organic Synthesis''; Elsevier: Burlington, 2005.Mitchell, T. N. ''J. Organomet. Chem.'', 1986, ''304'', 1–16.Mitchell, T. N. ''Synthesis'', 1992, 803–815. ()Farina, V. ''Pure Appl. Chem.'', 1996, ''68'', 73–78. ().Farina, V.; Krishnamurthy, V.; Scott, W. J. ''The Stille Reaction''; Wiley: Online, 2004. ().Espinet, P.; Echavarren, A. M. ''Angew. Chem. Int. Ed.'', 2004, ''43'', 4704–4734.()Pattenden, G.; Sinclair, D. J. ''J.Organomet. Chem.'', 2002, 653, 261–268.Kosugi, M.; Fugami, K. ''J. Organomet. Chem.'', 2002, 19, 10–16.Pierre Genet, J.; Savignac, M. ''J. Organomet. Chem.'', 1999, 576, 305–317.Cordova, C.; Bartolomé, C.; Martínez-Ilarduya, J.M..; Espinet, P. ''ACS Catal''., 2015, ''5'', 3040–3053.().
History
The first example of a palladium catalyzed coupling of aryl halides with organotin reagents was reported by Colin Eaborn in 1976.Azarian, D.; Dua, S. S.; Eaborn, C.; Walton, D. R. M. ''J. Organomet. Chem.'', 1976, ''117'', C55-C57. () This reaction yielded from 7% to 53% of diaryl product. This process was expanded to the coupling ofacyl chlorides
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
with alkyl-tin reagents in 1977 by Toshihiko Migita, yielding 53% to 87% ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
product.Kosugi, M.; Shimizu, Y.; Migita, T. ''Chem. Lett.'', 1977, ''6'', 1423–1424. ()
 In 1977, Migita published further work on the coupling of
In 1977, Migita published further work on the coupling of allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
-tin reagents with both aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
(C) and acyl (D) halides. The greater ability of allyl groups to migrate to the palladium catalyst allowed the reactions to be performed at lower temperatures. Yields for aryl halides ranged from 4% to 100%, and for acyl halides from 27% to 86%.Kosugi, M.; Sasazawa, K.; Shikizu, Y.; Migita, T. ''Chem. Lett.'', 1977, ''6'', 301–302. ()Kosugi, M.; Shimizu, Y.; Migita, T. ''J. Organomet. Chem.'', 1977, ''129'', C36-C38. () Reflecting the early contributions of Migita and Kosugi, the Stille reaction is sometimes called the Migita–Kosugi–Stille coupling.
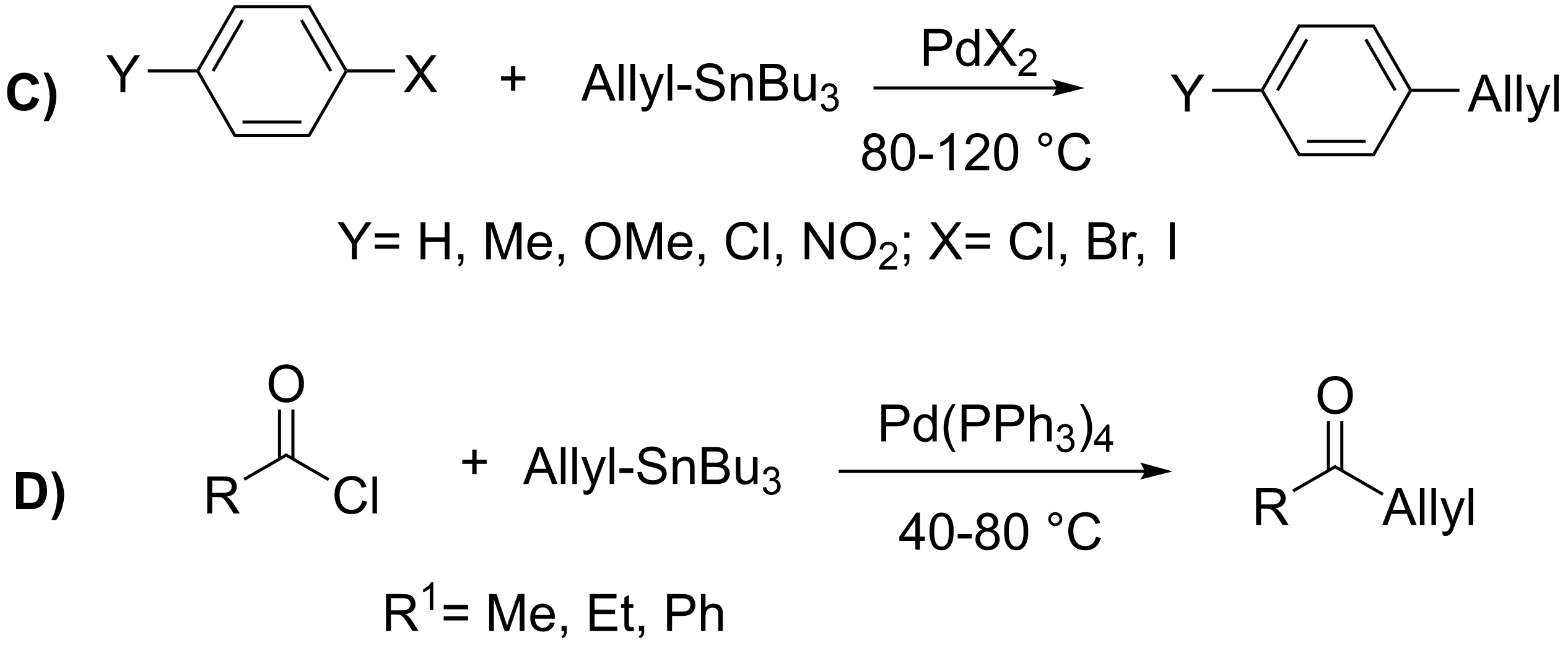 John Kenneth Stille subsequently reported the coupling of a variety of alkyl tin reagents in 1978 with numerous aryl and acyl halides under mild reaction conditions with much better yields (76%–99%).Milstein, D.; Stille, J. K. ''
John Kenneth Stille subsequently reported the coupling of a variety of alkyl tin reagents in 1978 with numerous aryl and acyl halides under mild reaction conditions with much better yields (76%–99%).Milstein, D.; Stille, J. K. ''Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'', 1978, ''100'', 3636–3638. () Stille continued his work in the 1980s on the synthesis of a multitude of ketones using this broad and mild process and elucidated a mechanism for this transformation.Milstein, D.; Stille, J. K. ''Journal of the American Chemical Society'', 1979, ''101'', 4992–4998. ()Milstein, D.; Stille, J. K. ''J. Org. Chem.'', 1979, ''44'', 1613–1618. ()
 By the mid-1980s, over 65 papers on the topic of coupling reactions involving tin had been published, continuing to explore the substrate scope of this reaction. While initial research in the field focused on the coupling of alkyl groups, most future work involved the much more synthetically useful coupling of
By the mid-1980s, over 65 papers on the topic of coupling reactions involving tin had been published, continuing to explore the substrate scope of this reaction. While initial research in the field focused on the coupling of alkyl groups, most future work involved the much more synthetically useful coupling of vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
, alkenyl, aryl, and allyl organostannanes to halides. Due to these organotin reagent's stability to air and their ease of synthesis, the Stille reaction became common in organic synthesis.
Mechanism
The mechanism of the Stille reaction has been extensively studied.Casado, A. L.; Espinet, P.; Gallego, A. M. ''J. Am, Chem. Soc.'', 2000, ''122'', 11771-11782. () The catalytic cycle involves an oxidative addition of ahalide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
or pseudohalide (2) to a palladium catalyst (1), transmetalation of 3 with an organotin reagent (4), and reductive elimination of 5 to yield the coupled product (7) and the regenerated palladium catalyst (1).Crabtree, R. H. ''The Organometallic Chemistry of the Transition Metals'', 5th ed.; Wiley: New York, 2009.
 However, the detailed mechanism of the Stille coupling is extremely complex and can occur via numerous reaction pathways. Like other palladium-catalyzed coupling reactions, the active palladium catalyst is believed to be a 14-electron Pd(0) complex, which can be generated in a variety of ways. Use of an 18- or 16- electron Pd(0) source , can undergo
However, the detailed mechanism of the Stille coupling is extremely complex and can occur via numerous reaction pathways. Like other palladium-catalyzed coupling reactions, the active palladium catalyst is believed to be a 14-electron Pd(0) complex, which can be generated in a variety of ways. Use of an 18- or 16- electron Pd(0) source , can undergo ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
dissociation to form the active species. Second, phosphines can be added to ligandless palladium(0). Finally, as pictured, reduction of a Pd(II) source (8) , , , , etc.) by added phosphine ligands or organotin reagents is also common
Oxidative addition
Oxidative addition to the 14-electron Pd(0) complex is proposed. This process gives a 16-electron Pd(II) species. It has been suggested that anionicligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ...
, such as OAc, accelerate this step by the formation of d(OAc)(PR3)nsup>−, making the palladium species more nucleophillic.Perez-Temprano, M. H.; Gallego, A. M.; Casares, J. A.; Espinet, P. ''Organometallics'', 2011, ''30'', 611–617. ().
In some cases, especially when an sp3-hybridized organohalide is used, an SN2 type mechanism tends to prevail, yet this is not as commonly seen in the literature.
However, despite normally forming a ''cis''-intermediate after a concerted oxidative addition, this product is in rapid equilibrium with its ''trans''-isomer.Minniti, D. ''Inorg. Chem'', 1994, ''33'', 2631–2634.().Casado, A. L.; Espinet, P. ''Organometallics'', 1998, ''17'', 954–959. ().
 There are multiple reasons why
There are multiple reasons why isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
is favored here. First, a bulky ligand set is usually used in these processes, such as phosphines, and it is highly unfavorable for them to adopt a ''cis'' orientation relative to each other, resulting in isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
to the more favorable trans product. An alternative explanation for this phenomenon, dubbed antisymbiosis or transphobia, is by invocation of the sdn model.Landis, C. R.; Firman, T. K.' Root, D. M.; Cleveland, T. ''Journal of the American Chemical Society'', 1998, ''120'', 1842–1854. (). Under this theory, palladium is a hypervalent species. Hence R1 and the trans ligand, being trans to each other, will compete with one palladium orbital for bonding. This 4-electron 3-center bond is weakest when two strong donating groups are present, which heavily compete for the palladium orbital. Relative to any ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
normally used, the C-donor R1 ligand has a much higher trans effect
In inorganic chemistry, the trans effect is the increased Lability#Chemistry, lability of ligands that are Cis-trans isomerism#Inorganic coordination complexes, trans to certain other ligands, which can thus be regarded as trans-directing ligands. ...
. This trans influence is a measure of how competitive ligands trans to each other will compete for palladium's orbital. The usual ligand set, phosphines, and C-donors (R1) are both soft ligands, meaning that they will form strong bonds to palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
, and heavily compete with each other for bonding.Vicente, J.; Arcas, A.; Bautista, D. ''Organometallics'', 1997, ''16'', 2127–2138. ().Pearson, R. G. ''Inorg. Chem'', 1973, ''12'', 712–713.(). Since halides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluo ...
or pseudohalides are significantly more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
, their bonding with palladium will be highly polarized, with most of the electron density
Electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial variables and is typical ...
on the X group, making them low trans effect
In inorganic chemistry, the trans effect is the increased Lability#Chemistry, lability of ligands that are Cis-trans isomerism#Inorganic coordination complexes, trans to certain other ligands, which can thus be regarded as trans-directing ligands. ...
ligands. Hence, it will be highly favorable for R1 to be trans to X, since the R1 group will be able to form a stronger bond to the palladium.
Transmetallation
Thetransmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
of the ''trans'' intermediate from the oxidative addition step is believed to proceed via a variety of mechanisms depending on the substrates and conditions. The most common type of transmetallation for the Stille coupling involves an associative mechanism. This pathway implies that the organostannane, normally a tin atom bonded to an allyl, alkenyl, or aryl group, can coordinate
In geometry, a coordinate system is a system that uses one or more numbers, or coordinates, to uniquely determine and standardize the position of the points or other geometric elements on a manifold such as Euclidean space. The coordinates are ...
to the palladium via one of these double bonds. This produces a fleeting pentavalent, 18-electron species, which can then undergo ligand detachment to form a square planar
In chemistry, the square planar molecular geometry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the co ...
complex again. Despite the organostannane being coordinated to the palladium through the R2 group, R2 must be formally transferred to the palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
(the R2-Sn bond must be broken), and the X group must leave with the tin, completing the transmetalation. This is believed to occur through two mechanisms.Garcia-Melchor, M.; Braga, A. A. C.; Lledos, A.; Ujaque, G.; Maseras, F. ''Acc. Chem. Res.'', 2013, ''46'', 2626–2634. ()
First, when the organostannane initially adds to the trans metal complex, the X group can coordinate
In geometry, a coordinate system is a system that uses one or more numbers, or coordinates, to uniquely determine and standardize the position of the points or other geometric elements on a manifold such as Euclidean space. The coordinates are ...
to the tin, in addition to the palladium, producing a cyclic transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
. Breakdown of this adduct results in the loss of R3Sn-X and a trivalent palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
complex with R1 and R2 present in a ''cis'' relationship. Another commonly seen mechanism involves the same initial addition of the organostannane to the ''trans'' palladium complex as seen above; however, in this case, the X group does not coordinate to the tin, producing an open transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
. After the α-carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
relative to tin attacks the palladium, the tin complex will leave with a net positive charge. In the scheme below, please note that the double bond coordinating to tin denotes R2, so any alkenyl, allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
, or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
group. Furthermore, the X group can dissociate at any time during the mechanism and bind to the Sn+ complex at the end. Density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
calculations predict that an open mechanism will prevail if the 2 ligands
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ...
remain attached to the palladium and the X group leaves, while the cyclic mechanism is more probable if a ligand dissociates prior to the transmetalation. Hence, good leaving groups such as triflates in polar solvents favor the cyclic transition state, while bulky phosphine ligands will favor the open transition state.
 A less common pathway for transmetalation is through a dissociative or solvent assisted mechanism. Here, a ligand from the tetravalent palladium species dissociates, and a coordinating solvent can add onto the palladium. When the
A less common pathway for transmetalation is through a dissociative or solvent assisted mechanism. Here, a ligand from the tetravalent palladium species dissociates, and a coordinating solvent can add onto the palladium. When the solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
detaches, to form a 14-electron trivalent intermediate, the organostannane can add to the palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
, undergoing an open or cyclic type process as above.
Reductive elimination step
In order for R1-R2 to reductively eliminate, these groups must occupy mutually ''cis'' coordination sites. Any ''trans''-adducts must therefore isomerize to the ''cis'' intermediate or the coupling will be frustrated. A variety of mechanisms exist for reductive elimination and these are usually considered to be concerted.Gillie, A.; Stille, J. K. ''Journal of the American Chemical Society'', 1980, ''102'', 4933–4941. ().Brown, J. M.; Cooley, N. A. ''Chem. Rev.'', 1988, ''88'', 1031–1046. (). First, the 16-electron tetravalent intermediate from the transmetalation step can undergo unassisted reductive elimination from asquare planar
In chemistry, the square planar molecular geometry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the co ...
complex. This reaction occurs in two steps: first, the reductive elimination is followed by coordination of the newly formed sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
between R1 and R2 to the metal, with ultimate dissociation yielding the coupled product.
 The previous process, however, is sometimes slow and can be greatly accelerated by dissociation of a ligand to yield a 14-electron T shaped intermediate. This intermediate can then rearrange to form a Y-shaped adduct, which can undergo faster reductive elimination.
The previous process, however, is sometimes slow and can be greatly accelerated by dissociation of a ligand to yield a 14-electron T shaped intermediate. This intermediate can then rearrange to form a Y-shaped adduct, which can undergo faster reductive elimination.
 Finally, an extra ligand can associate to the palladium to form an 18-electron trigonal bipyramidal structure, with R1 and R2 cis to each other in equatorial positions. The geometry of this intermediate makes it similar to the Y-shaped above.
Finally, an extra ligand can associate to the palladium to form an 18-electron trigonal bipyramidal structure, with R1 and R2 cis to each other in equatorial positions. The geometry of this intermediate makes it similar to the Y-shaped above.
 The presence of bulky ligands can also increase the rate of elimination. Ligands such as phosphines with large bite angles cause steric repulsion between L and R1 and R2, resulting in the angle between L and the R groups to increase and the angle between R1 and R2 to hence decrease, allowing for quicker reductive elimination.
The presence of bulky ligands can also increase the rate of elimination. Ligands such as phosphines with large bite angles cause steric repulsion between L and R1 and R2, resulting in the angle between L and the R groups to increase and the angle between R1 and R2 to hence decrease, allowing for quicker reductive elimination.

Kinetics
The rate at which organostannanes transmetalate with palladium catalysts is shown below. Sp2-hybridized carbon groups attached to tin are the most commonly used coupling partners, and sp3-hybridized carbons require harsher conditions and terminal alkynes may be coupled via a C-H bond through the Sonogashira reaction. As the organic tin compound, a trimethylstannyl or tributylstannyl compound is normally used. Although trimethylstannyl compounds show higher reactivity compared with tributylstannyl compounds and have much simpler 1H-NMR spectra, the toxicity of the former is much larger.
Optimizing which ligands are best at carrying out the reaction with high yield and turnover rate can be difficult. This is because the oxidative addition requires an electron rich metal, hence favoring electron donating ligands. However, an electron deficient metal is more favorable for the transmetalation and reductive elimination steps, making electron withdrawing ligands the best here. Therefore, the optimal ligand set heavily depends on the individual substrates and conditions used. These can change the rate determining step, as well as the mechanism for the transmetalation step.Farina, V.; ''Journal of the American Chemical Society'', 1991, ''113'', 9585–9595. ().
Normally, ligands of intermediate donicity, such as phosphines, are utilized. Rate enhancements can be seen when moderately electron-poor ligands, such as tri-2-furylphosphine or triphenylarsenine are used. Likewise, ligands of high donor number can slow down or inhibit coupling reactions.
These observations imply that normally, the rate-determining step for the Stille reaction is transmetalation.
As the organic tin compound, a trimethylstannyl or tributylstannyl compound is normally used. Although trimethylstannyl compounds show higher reactivity compared with tributylstannyl compounds and have much simpler 1H-NMR spectra, the toxicity of the former is much larger.
Optimizing which ligands are best at carrying out the reaction with high yield and turnover rate can be difficult. This is because the oxidative addition requires an electron rich metal, hence favoring electron donating ligands. However, an electron deficient metal is more favorable for the transmetalation and reductive elimination steps, making electron withdrawing ligands the best here. Therefore, the optimal ligand set heavily depends on the individual substrates and conditions used. These can change the rate determining step, as well as the mechanism for the transmetalation step.Farina, V.; ''Journal of the American Chemical Society'', 1991, ''113'', 9585–9595. ().
Normally, ligands of intermediate donicity, such as phosphines, are utilized. Rate enhancements can be seen when moderately electron-poor ligands, such as tri-2-furylphosphine or triphenylarsenine are used. Likewise, ligands of high donor number can slow down or inhibit coupling reactions.
These observations imply that normally, the rate-determining step for the Stille reaction is transmetalation.
Additives
The most common additive to the Stille reaction isstoichiometric
Stoichiometry () is the relationships between the masses of reactants and products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must equal the total m ...
or co-catalytic copper(I), specifically copper iodide, which can enhance rates
Rate or rates may refer to:
Finance
* Rate (company), an American residential mortgage company formerly known as Guaranteed Rate
* Rates (tax), a type of taxation system in the United Kingdom used to fund local government
* Exchange rate, rate ...
up by >103 fold. It has been theorized that in polar solvents
A solvent (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
transmetalate with the organostannane. The resulting organocuprate reagent could then transmetalate with the palladium catalyst. Furthermore, in ethereal solvents, the copper could also facilitate the removal of a phosphine ligand, activating the Pd center.Liebeskind, L. S.; Fengl, R. W. ''J. Org. Chem.'', 1990, ''55'', 5359–5364. ().Farina, V.; Kapadia, S.; Brishnan, B.; Wang, C.; Liebeskind, L. S. ''J, Org. Chem'', 1994, ''59'', 5905–5911. ().Mee, S. P. H.; Lee, V.; Baldwin, J. E. ''Angew. Chem. Int. Ed.'', 2004, ''43'', 1132–1136.Liebeskind, L. S.; Peña-Cabrera, E. ''Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
'', Coll. Vol. 10, p. 9 (2004); Vol. 77, p. 135 (2000).Article
Lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorid ...
has been found to be a powerful rate accelerant in cases where the X group dissociates from palladium (i.e. the open mechanism). The chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
ion is believed to either displace the X group on the palladium making the catalyst more active for transmetalation or by coordination to the Pd(0) adduct to accelerate the oxidative addition. Also, LiCl salt enhances the polarity of the solvent, making it easier for this normally anionic ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
(– Cl, – Br, – OTf, etc.) to leave. This additive is necessary when a solvent like THF is used; however, utilization of a more polar solvent, such as NMP, can replace the need for this salt additive. However, when the coupling's transmetalation step proceeds via the cyclic mechanism, addition of lithium chloride can actually decrease the rate. As in the cyclic mechanism, a neutral ligand, such as phosphine, must dissociate instead of the anionic X group.Scott, W. J.; Stille, J. K. ''Journal of the American Chemical Society'', 1986, ''108'', 3033–3040. ().
Finally, sources of fluoride ions, such as cesium fluoride, also effect on the catalytic cycle. First, fluoride can increase the rates of reactions of organotriflates, possibly by the same effect as lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorid ...
. Furthermore, fluoride ions can act as scavengers
Scavengers are animals that consume dead organisms that have died from causes other than predation or have been killed by other predators. While scavenging generally refers to carnivores feeding on carrion, it is also a herbivorous feeding ...
for tin byproducts, making them easier to remove via filtration
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a ''filter medium'' that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filte ...
.
Competing side reactions
The most common side reactivity associated with the Stille reaction is homocoupling of the stannane reagents to form an R2-R2 dimer. It is believed to proceed through two possible mechanisms. First, reaction of two equivalents of organostannane with the Pd(II) precatalyst will yield the homocoupled product after reductive elimination. Second, the Pd(0) catalyst can undergo a radical process to yield the dimer. The organostannane reagent used is traditionally tetravalent at tin, normally consisting of the sp2-hybridized group to be transferred and three "non-transferable"alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
groups. As seen above, alkyl groups are normally the slowest at migrating onto the palladium catalyst.
 It has also been found that at temperatures as low as 50 °C,
It has also been found that at temperatures as low as 50 °C, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
groups on both palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
and a coordinated phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
can exchange. While normally not detected, they can be a potential minor product in many cases.
side reaction
A side reaction is a chemical reaction that occurs at the same time as the actual main reaction, but to a lesser extent. It leads to the formation of by-product, so that the Yield (chemistry), yield of main product is reduced:
: + B ->[] P1
: + C ...
is known as Arene substitution pattern#Cine and tele substitution, cine substitution. Here, after initial oxidative addition of an aryl halide, this Pd-Ar species can insert across a vinyl tin double bond. After β-hydride elimination, migratory insertion, and protodestannylation, a 1,2-disubstituted olefin can be synthesized.
hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
, yet when very electron-rich aryl stannanes are used, this can become a significant side reaction.
Scope
Electrophile
Vinyl halides are common coupling partners in the Stille reaction, and reactions of this type are found in numerousnatural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
total syntheses. Normally, vinyl iodides and bromides are used. Vinyl chlorides are insufficiently reactive toward oxidative addition to Pd(0). Iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
s are normally preferred: they will typically react faster and under milder conditions than will bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
s. This difference is demonstrated below by the selective coupling of a vinyl iodide in the presence of a vinyl bromide.
stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
of the alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
is retained throughout the reaction, except under harsh reaction conditions. A variety of alkenes may be used, and these include both α- and β-halo-α,β unsaturated ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s, ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s, and sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s (which normally need a copper (I) additive to proceed), and more (see example below).Johnson, C. R.; Adams, J. P.; Braun, M.P.; Senanayake, C. B. W. ''Tetrahedron Lett''., 1992, ''33'', 919–922. () Vinyl triflates are also sometimes used. Some reactions require the addition of LiCl and others are slowed down, implying that two mechanistic pathways are present.
 Another class of common electrophiles are aryl and
Another class of common electrophiles are aryl and heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
halides. As for the vinyl substrates, bromides and iodides are more common despite their greater expense. A multitude of aryl groups can be chosen, including rings substituted with electron donating substituents, biaryl rings, and more. Halogen-substituted heterocycles have also been used as coupling partners, including pyridines, furans, thiophenes
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromaticity, aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most ...
, thiazoles, indoles
Indole is an organic compound with the formula . Indole is classified as an aromatic Heterocyclic compound, heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are d ...
, imidazoles, purines
Purine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which inc ...
, uracil
Uracil () (nucleoside#List of nucleosides and corresponding nucleobases, symbol U or Ura) is one of the four nucleotide bases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via ...
, cytosine
Cytosine () (symbol C or Cyt) is one of the four nucleotide bases found in DNA and RNA, along with adenine, guanine, and thymine ( uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attac ...
s, pyrimidines
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
, and more (See below for table of heterocycles; halogens can be substituted at a variety of positions on each).
 Below is an example of the use of Stille coupling to build complexity on heterocycles of
Below is an example of the use of Stille coupling to build complexity on heterocycles of nucleosides
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar (ribose or 2'-deoxyribose) whereas a nucleotide ...
, such as purines
Purine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which inc ...
.Nair, V.; Turner, G. A.; Chamberlain, S. D. ''Journal of the American Chemical Society'', 1987, ''109'', 7223–7224. ().
 Aryl triflates and
Aryl triflates and sulfonates
In organosulfur chemistry, a sulfonate is a salt, anion or ester of a sulfonic acid. Its formula is , containing the functional group , where R is typically an organyl group, amino group or a halogen atom. Sulfonates are the conjugate bases of ...
are also couple to a wide variety of organostannane reagents. Triflates tend to react comparably to bromides in the Stille reaction.
Acyl chlorides
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
are also used as coupling partners and can be used with a large range of organostannane, even alkyl-tin reagents, to produce ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
(see example below).Jousseaume, B.; Kwon, W.; Verlhac, J. B.; Denat, F.; Dubac, J. ''Synlett'', 1993, 117–118. () However, it is sometimes difficult to introduce acyl chloride functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
into large molecules with sensitive functional groups. An alternative developed to this process is the Stille-carbonylative cross-coupling reaction, which introduces the carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
group via carbon monoxide insertion.
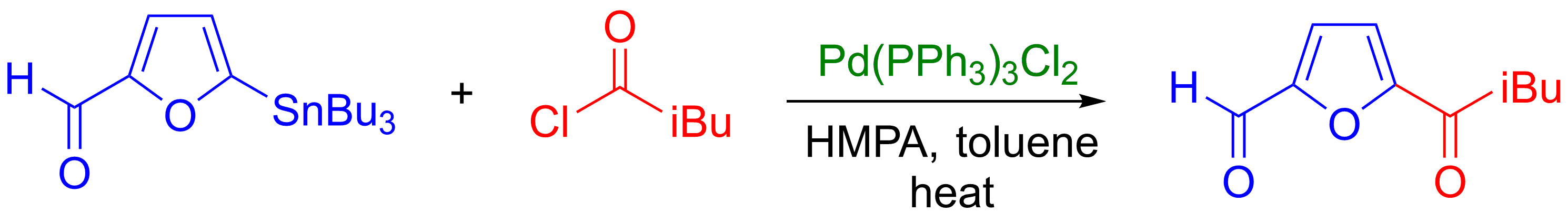
Allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolat ...
, benzylic, and propargylic
In organic chemistry, the propargyl group is a functional group of 2- propynyl with the structure . It is an alkyl group derived from propyne ().
The term propargylic refers to a saturated position ( ''sp''3-hybridized) on a molecular framewor ...
halides can also be coupled. While commonly employed, allylic halides proceed via an η3 transition state, allowing for coupling with the organostannane at either the α or γ position, occurring predominantly at the least substituted carbon (see example below).Sheffy, F. K.; Godschalx, J. P.; Stille, J. K. ''Journal of the American Chemical Society'', 1984, ''106'', 4833–4840. () Alkenyl epoxides (adjacent epoxides
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom Ring (chemistry), ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly Reactivity ( ...
and alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
) can also undergo this same coupling through an η3 transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
as, opening the epoxide to an alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
. While allylic and benzylic acetates
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called ...
are commonly used, propargylic acetates are unreactive with organostannanes.

Stannane
Organostannane reagents are common. Several are commercially available. Stannane reagents can be synthesized by the reaction of a Grignard ororganolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
with trialkyltin chlorides. For example, vinyltributyltin is prepared by the reaction of vinylmagnesium bromide with tributyltin chloride. Hydrostannylation of alkynes
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
or alkenes
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
provides many derivatives. Organotin reagents are air and moisture stable. Some reactions can even take place in water.Wolf, C.; Lerebours, R. ''J. Org. Chem.'', 2003,''68'' 7551–7554. (). They can be purified by chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
. They are tolerant to most functional groups. Some organotin compounds are heavily toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
, especially trimethylstannyl derivatives.
The use of vinylstannane, or alkenylstannane reagents is widespread. In regards to limitations, both very bulky stannane reagents and stannanes with substitution on the α-carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
tend to react sluggishly or require optimization. For example, in the case below, the α-substituted vinylstannane only reacts with a terminal iodide due to steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
.Crisp, G.T.; Glink, P. T. ''Tetrahedron'', 1994, ''50'', 2623. ()
 Arylstannane reagents are also common and both electron donating and electron withdrawing groups actually increase the rate of the transmetalation. This again implies that two mechanisms of transmetalation can occur. The only limitation to these reagents are substituents at the ortho-position as small as methyl groups can decrease the rate of reaction. A wide variety of heterocycles (see Electrophile section) can also be used as coupling partners (see example with a thiazole ring below).Bailey, T. R. ''Tetrahedron Lett''., 1986, ''27'', 4407. ().
Arylstannane reagents are also common and both electron donating and electron withdrawing groups actually increase the rate of the transmetalation. This again implies that two mechanisms of transmetalation can occur. The only limitation to these reagents are substituents at the ortho-position as small as methyl groups can decrease the rate of reaction. A wide variety of heterocycles (see Electrophile section) can also be used as coupling partners (see example with a thiazole ring below).Bailey, T. R. ''Tetrahedron Lett''., 1986, ''27'', 4407. ().

 Alkynylstannanes, the most reactive of stannanes, have also been used in Stille couplings. They are not usually needed as terminal alkynes can couple directly to palladium catalysts through their C-H bond via
Alkynylstannanes, the most reactive of stannanes, have also been used in Stille couplings. They are not usually needed as terminal alkynes can couple directly to palladium catalysts through their C-H bond via Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
. Allylstannanes have been reported to have worked, yet difficulties arise, like with allylic halides, with the difficulty in control regioselectivity for α and γ addition. Distannane and acyl stannane reagents have also been used in Stille couplings.
Applications
The Stille reaction has been used in the synthesis of a variety of polymers.Bao, Z.; Chan, W.; Yu, L. ''Chem. Mater.'', 1993, ''5'', 2–3. ().Bao, Z.; Chan, W. K.; Yu, L. ''Journal of the American Chemical Society'', 1995, ''117'', 12426-12435. ().Sun, S. S.; Lewis, J. E.; Zhang, J.; Jiang, X.; Zhang, C.; Matos, T.; Li, R.; ''Polym. Chem.'', 2010, ''1'', 663–669. () However, the most widespread use of the Stille reaction is its use inorganic syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
, and specifically, in the synthesis of natural products
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
.
Natural product total synthesis
Larry Overman's 19-step enantioselectivetotal synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
of quadrigemine C involves a double Stille cross metathesis reaction.Lebsack, A. D.; Link, J. T.; Overman, L. E.; Stearns, B. A. ''Journal of the American Chemical Society'', 2002, ''124'', 9008–9009. () The complex organostannane is coupled onto two aryl iodide groups. After a double Heck cyclization, the product is achieved.
 Panek's 32 step enantioselective
Panek's 32 step enantioselective total synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
of ansamycin antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
(+)-mycotrienol makes use of a late stage tandem Stille type macrocycle coupling. Here, the organostannane has two terminal tributyl tin groups attacked to an alkene. This organostannane "stitches" the two ends of the linear starting material into a macrocycle, adding the missing two methylene units in the process. After oxidation of the aromatic core with ceric ammonium nitrate (CAN) and deprotection with hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
yields the natural product in 54% yield for the 3 steps.Masse, C. E.; Yang, M.; Solomon, J.; Panek, J. S. ''Journal of the American Chemical Society'', 1998, ''120'', 4123–4134. ()
 Stephen F. Martin and coworkers' 21 step enantioselective total synthesis of the manzamine antitumor alkaloid Ircinal A makes use of a tandem one-pot Stille/Diels-Alder reaction. An alkene group is added to vinyl bromide, followed by an ''in situ'' Diels-Alder cycloaddition between the added alkene and the alkene in the pyrrolidine ring.Martin, S. F.; Humphrey, J. M.; Ali, A.; Hillier, M. C. ''Journal of the American Chemical Society'', 1999, ''121'', 866–867. ()
Stephen F. Martin and coworkers' 21 step enantioselective total synthesis of the manzamine antitumor alkaloid Ircinal A makes use of a tandem one-pot Stille/Diels-Alder reaction. An alkene group is added to vinyl bromide, followed by an ''in situ'' Diels-Alder cycloaddition between the added alkene and the alkene in the pyrrolidine ring.Martin, S. F.; Humphrey, J. M.; Ali, A.; Hillier, M. C. ''Journal of the American Chemical Society'', 1999, ''121'', 866–867. ()
 Numerous other total syntheses utilize the Stille reaction, including those of oxazolomycin,Kende, A. S.; Kawamura, K.; DeVita, R. J. ''Journal of the American Chemical Society'', 1990, ''112'' 4070–4072. (). lankacidin C,Kende, A. S., Koch, K.; Dorey, G.; Kaldor, I.; Liu, K. ''Journal of the American Chemical Society'', 1993, ''115'', 9842–9843. (). onamide A,Hong, C. Y, Kishi, Y. ''Journal of the American Chemical Society'', 1991, ''113'', 9693–9694. (). calyculin A,Tanimoto, N.; Gerritz, S. W.; Sawabe, A.; Noda, T.; Filla, S. A.; Masamune, S. ''Angew. Chem. Int. Ed.'', 2003, ''33'', 673–675. (). lepicidin A,Evans, D. A.; Black, W. C. ''Journal of the American Chemical Society'', 1993, ''115'', 4497–4513. (). ripostatin A,Tang, W.; Prusov, E. V. ''Org. Lett.'', 2012, ''14'' 4690–4693. (). and lucilactaene.Coleman, R. S.; Walczak, M. C.; Campbell, E. L. ''Journal of the American Chemical Society'', 2005, ''127'', 16036-16039. (). The image below displays the final
Numerous other total syntheses utilize the Stille reaction, including those of oxazolomycin,Kende, A. S.; Kawamura, K.; DeVita, R. J. ''Journal of the American Chemical Society'', 1990, ''112'' 4070–4072. (). lankacidin C,Kende, A. S., Koch, K.; Dorey, G.; Kaldor, I.; Liu, K. ''Journal of the American Chemical Society'', 1993, ''115'', 9842–9843. (). onamide A,Hong, C. Y, Kishi, Y. ''Journal of the American Chemical Society'', 1991, ''113'', 9693–9694. (). calyculin A,Tanimoto, N.; Gerritz, S. W.; Sawabe, A.; Noda, T.; Filla, S. A.; Masamune, S. ''Angew. Chem. Int. Ed.'', 2003, ''33'', 673–675. (). lepicidin A,Evans, D. A.; Black, W. C. ''Journal of the American Chemical Society'', 1993, ''115'', 4497–4513. (). ripostatin A,Tang, W.; Prusov, E. V. ''Org. Lett.'', 2012, ''14'' 4690–4693. (). and lucilactaene.Coleman, R. S.; Walczak, M. C.; Campbell, E. L. ''Journal of the American Chemical Society'', 2005, ''127'', 16036-16039. (). The image below displays the final natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
, the organohalide (blue), the organostannane (red), and the bond being formed (green and circled). From these examples, it is clear that the Stille reaction can be used both at the early stages of the synthesis (oxazolomycin and calyculin A), at the end of a convergent route (onamide A, lankacidin C, ripostatin A), or in the middle (lepicidin A and lucilactaene). The synthesis of ripostatin A features two concurrent Stille couplings followed by a ring-closing metathesis
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various Saturated and unsaturated compounds, unsaturated rings via the intramolecular olefin metathesis, metathesis of two term ...
. The synthesis of lucilactaene features a middle subunit, having a borane on one side and a stannane on the other, allowing for a Stille reaction followed by a subsequent Suzuki coupling.

Variations
In addition to performing the reaction in a variety of organic solvents, conditions have been devised which allow for a broad range of Stille couplings in aqueous solvent. In the presence of Cu(I) salts, palladium-on-carbon has been shown to be an effective catalyst.Roth, G. P.; Farina, V.; Liebeskind, L. S.; Peña-Cabrera, E. '' Tetrahedron Lett.'' 1995, ''36'', 2191.Renaldo, A. F.; Labadie, J. W.; Stille, J. K. ''Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
'', Coll. Vol. 8, p. 268 (1993); Vol. 67, p. 86 (1989).Article
In the realm of
green chemistry
Green chemistry, similar to sustainable chemistry or circular chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize or eliminate the use and generation of hazardous substances. Wh ...
a Stille reaction is reported taking place in a low melting and highly polar mixture of a sugar such as mannitol
Mannitol is a type of sugar alcohol used as a sweetener and medication. It is used as a low calorie sweetener as it is poorly absorbed by the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to l ...
, a urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
such as dimethylurea and a salt such as ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
''Stille Reactions with Tetraalkylstannanes and Phenyltrialkylstannanes in Low Melting Sugar-Urea-Salt Mixtures''Giovanni Imperato, Rudolf Vasold, Burkhard König Advanced Synthesis & Catalysis Volume 348, Issue 15 , Pages 2243–47 2006
. The catalyst system is with triphenylarsine:
Stille–carbonylative cross-coupling
A common alteration to the Stille coupling is the incorporation of acarbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
group between R1 and R2, serving as an efficient method to form ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s. This process is extremely similar to the initial exploration by Migita and Stille (see History) of coupling organostannane to acyl chlorides
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
. However, these moieties are not always readily available and can be difficult to form, especially in the presence of sensitive functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
. Furthermore, controlling their high reactivity can be challenging. The Stille-carbonylative cross-coupling employs the same conditions as the Stille coupling, except with an atmosphere of carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
(CO) being used. The CO can coordinate to the palladium catalyst (9) after initial oxidative addition, followed by CO insertion into the Pd-R1 bond (10), resulting in subsequent reductive elimination to the ketone (12). The transmetalation step is normally the rate-determining step.
 Larry Overman and coworkers make use of the Stille-carbonylative cross-coupling in their 20-step enantioselective
Larry Overman and coworkers make use of the Stille-carbonylative cross-coupling in their 20-step enantioselective total synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
of strychnine
Strychnine (, , American English, US chiefly ) is a highly toxicity, toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, ...
. The added carbonyl is later converted to a terminal alkene via a Wittig reaction, allowing for the key tertiary nitrogen and the pentacyclic core to be formed via an aza-Cope
A cope ( ("rain coat") or ("cape")) is a liturgical long mantle or cloak, open at the front and fastened at the breast with a band or clasp. It may be of any liturgical colour.
A cope may be worn by any rank of the Catholic or Anglican clerg ...
- Mannich reaction.Knight, S. D.; Overman, L. E.; Pairaudeau, G. ''Journal of the American Chemical Society'', 1993, ''115'', 9293–9294. ()
 Giorgio Ortar et al. explored how the Stille-carbonylative cross-coupling could be used to synthesize
Giorgio Ortar et al. explored how the Stille-carbonylative cross-coupling could be used to synthesize benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
phosphores. These were embedded into 4-benzoyl-L-phenylalanine peptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Dalton (unit), Da or more are called proteins. Chains of fewer t ...
and used for their photoaffinity labelling properties to explore various peptide-protein interactions.Monera, E.; Ortar, G. ''Biorg. Med. Chem. Lett.'', 2000, ''10'', 1815–1818. ().
 Louis Hegedus' 16-step
Louis Hegedus' 16-step racemic
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as r ...
total synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
of Jatraphone involved a Stille-carbonylative cross-coupling as its final step to form the 11-membered macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. ...
. Instead of a halide, a vinyl triflate is used there as the coupling partner.Gyorkos, A. C.; Stille, J. K.; Hegedus, L. S. ''Journal of the American Chemical Society'', 1990, ''112'', 8465–8472. ().

Stille–Kelly coupling
Using the seminal publication by Eaborn in 1976, which forms arylstannanes from arylhalides and distannanes, T. Ross Kelly applied this process to the intramolecular coupling of arylhalides. This tandem stannylation/aryl halide coupling was used for the syntheses of a variety of dihydrophenanthrenes. Most of the internal rings formed are limited to 5 or 6 members, however some cases of macrocyclization have been reported. Unlike a normal Stille coupling, chlorine does not work as a halogen, possibly due to its lower reactivity in thehalogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
sequence (its shorter bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
and stronger bond dissociation energy makes it more difficult to break via oxidative addition). Starting in the middle of the scheme below and going clockwise, the palladium catalyst (1) oxidatively adds to the most reactive C-X bond (13) to form 14, followed by transmetalation with distannane (15) to yield 16 and reductive elimination to yield an arylstannane (18). The regenerated palladium catalyst (1) can oxidative add to the second C-X bond of 18 to form 19, followed by intramolecular transmetalation to yield 20, followed by reductive elimination to yield the coupled product (22).
 Jie Jack Lie et al. made use of the Stille-Kelly coupling in their synthesis of a variety of benzo ,5uropyridines ring systems. They invoke a three-step process, involving a Buchwald-Hartwig amination, another palladium-catalyzed coupling reaction, followed by an intramolecular Stille-Kelly coupling. Note that the aryl-iodide bond will oxidatively add to the
Jie Jack Lie et al. made use of the Stille-Kelly coupling in their synthesis of a variety of benzo ,5uropyridines ring systems. They invoke a three-step process, involving a Buchwald-Hartwig amination, another palladium-catalyzed coupling reaction, followed by an intramolecular Stille-Kelly coupling. Note that the aryl-iodide bond will oxidatively add to the palladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
faster than either of the aryl-bromide bonds.Yue, W. S.; Li, J. J. ''Org. Lett.'', 2002, ''4'', 2201–2203. ()

See also
*Organotin chemistry
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discove ...
* Organostannane addition
* Palladium-catalyzed coupling reactions
*Suzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
* Negishi coupling
* Heck reaction
* Hiyama coupling
References
{{reflistExternal links
Stille reaction handout
from the Myers group.
Stille reaction
at organic-chemistry.org Carbon-carbon bond forming reactions Palladium Name reactions