|
Acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic, or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminium'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic anion , or . Most of the approximately 5 million tonnes of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common building block for biosynthesis. Nomenclature and common formula When part of a salt, the formula of the acetate i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. Production and synthesis Ethyl acetate was first synthesized by the Louis-Léon de Brancas, Count de Lauraguais in 1759 by distilling a mixture of ethanol and acetic acid. In 2004, an estimated 1.3 million tonnes were produced worldwide. The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tonnes. The global ethyl acetate market was valued at $3.3 billion in 2018. Ethyl acetate is produced in industry mainly via the classic Fischer esterification reaction of ethanol and acetic acid. This mixture converts to the ester in about 65% ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
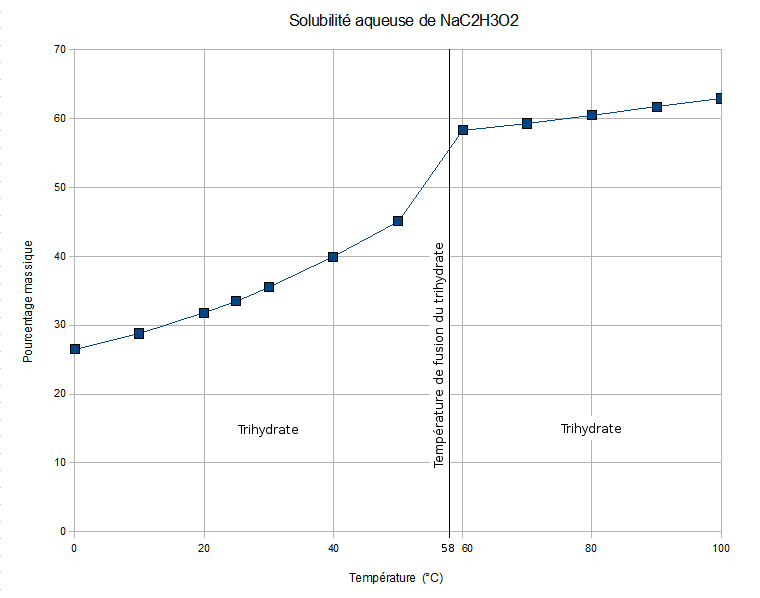
Sodium Acetate
Sodium acetate, CH3COONa, also abbreviated Sodium, NaOxygen, OAcetyl, Ac, is the sodium Salt (chemistry), salt of acetic acid. This salt is colorless, deliquescent, and hygroscopy, hygroscopic. Applications Biotechnological Sodium acetate is used as the carbon source (biology) , carbon source for culturing Bacterial growth, bacteria. Sodium acetate can also be useful for increasing yields of DNA extraction, DNA isolation by ethanol precipitation. Industrial Sodium acetate is used in the textile industry to neutralize sulfuric acid waste streams and also as a photoresist while using aniline dyes. It is also a pickling (metal), pickling agent in chrome Tanning (leather), tanning and helps to impede vulcanization of chloroprene in synthetic rubber production. It is also used to reduce static electricity during production of disposable cotton pads. Concrete longevity Sodium acetate is used as a sealant to mitigate water damage to concrete. It is environm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is funda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Acetate
Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the chemical formula, formula Cr2(CH3CO2)4(H2O)2. This formula is commonly abbreviated Cr2(OAc)4(H2O)2. This red-coloured compound features a quadruple bond. It exists as the dihydrate and the anhydrous forms. Both are diamagnetic. Cr2(OAc)4(H2O)2 is a reddish diamagnetic powder, although diamond-shaped tabular crystals can be grown. Consistent with the fact that it is nonionic, Cr2(OAc)4(H2O)2 exhibits poor solubility in water and methanol. Structure The Cr2(OAc)4(H2O)2 molecule contains two atoms of chromium, two ligand, ligated molecules of water, and four acetate bridging ligands. The coordination environment around each chromium atom consists of four oxygen atoms (one from each acetate ligand) in a square, one water molecule (in an axial position), and the other chromium atom (opposite the water molecule), giving each chromium centre an Octahedral molecular geometry, octahedral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Acetate
Aluminium acetate or aluminium ethanoate (also "aluminum ~"), sometimes abbreviated AlAc in geochemistry, can refer to a number of different salts of aluminium with acetic acid. In the solid state, three salts exist under this name: basic aluminium monoacetate, (HO)2AlCH3CO2, basic aluminium diacetate, HOAl(CH3CO2)2, and neutral aluminium triacetate, Al(CH3CO2)3. In aqueous solution, aluminium triacetate hydrolyses to form a mixture of the other two, and all solutions of all three can be referred to as "aluminium acetate" as the species formed co-exist and inter-convert in chemical equilibrium. Stoichiometry Monoacetate Aluminium monoacetate, also known as dibasic aluminium acetate, forms from Al(OH)3 and dilute aqueous acetic acid. More concentrated acid leads to the di- and triacetate. Diacetate Aluminium diacetate, also known as basic aluminium acetate, is prepared from aqueous aluminium acetate solution resulting in a white powder. This basic salt forms from the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions ( cations) and negatively charged ions ( anions), which results in a compound with no net electric charge (electrically neutral). The constituent ions are held together by electrostatic forces termed ionic bonds. The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic, such as ammonium () and carbonate () ions in ammonium carbonate. Salts containing basic ions hydroxide (OH−) or oxide (O2−) are classified as bases, such as sodium hydroxide and potassium oxide. Individual ions within a salt usually have multiple near neighbours, so they are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Salts usually form crystalline structures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymers
A polymer () is a substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. Polymers are studied in the fields of polymer science (which includes polymer chemistry and polymer p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve as enzyme substrate (chemistry), substrates, with conversion by the living organism either into simpler or more complex Product (chemistry), products. Examples of biosynthetic pathways include those for the production of amino acids, lipid membrane components, and nucleotides, but also for the production of all classes of biological macromolecules, and of acetyl-coenzyme A, adenosine triphosphate, nicotinamide adenine dinucleotide and other key intermediate and transactional molecules needed for metabolism. Thus, in biosynthesis, any of an array of Chemical compound, compounds, from simple to complex, are converted into other compounds, and so it includes both the catabolism and anabolism (building up and breaking down) of comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... Carboxylate esters have the general formula (also written as ), where R and R′ are organic groups. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. : Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actinium
Actinium is a chemical element; it has chemical symbol, symbol Ac and atomic number 89. It was discovered by Friedrich Oskar Giesel in 1902, who gave it the name ''emanium''; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. The actinide series, a set of 15 elements between actinium and lawrencium in the periodic table, are named for actinium. Together with polonium, radium, and radon, actinium was one of the first Primordial element, non-primordial radioactive elements to be discovered. A soft, silvery-white radioactive metal, actinium reacts rapidly with oxygen and moisture in air forming a white coating of actinium oxide that prevents further oxidation. As with most lanthanides and many actinides, actinium assumes oxidation state +3 in nearly all its chemical compounds. Actinium is found only in traces in uranium and thorium ores as the isotope 227Ac, which decays with a half-life of 21.772 years, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |