|
Tributyltin Chloride
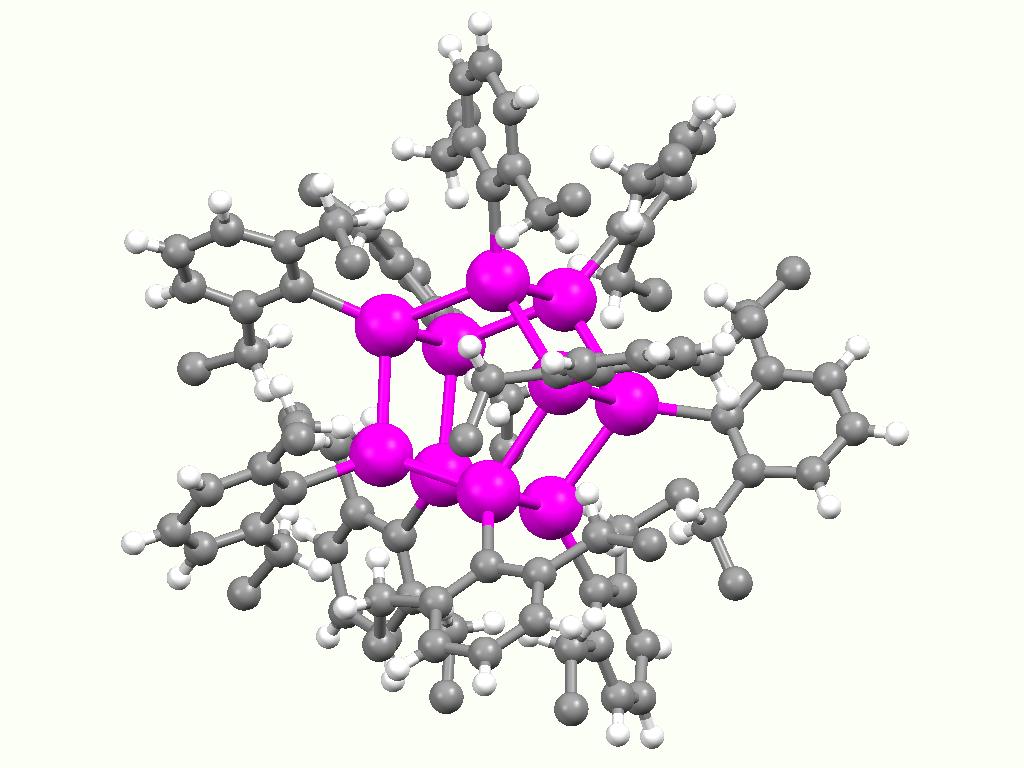
Tributyltin chloride is an organotin compound with the formula ( C4H9)3SnCl. It is a colorless liquid that is soluble in organic solvents. Preparation and reactions The compound is prepared by a redistribution reaction by combining stannic chloride and tetrabutyltin: :3 (C4H9)4Sn + SnCl4 → 4 (C4H9)3SnCl Tributyltin chloride hydrolyzes to the oxide C4H9)3Snsub>2O Tributyltin chloride is used as a precursor to other organotin compounds{{cite journal, title=Palladium-catalyzed Coupling Of Acid Chlorides With Organotin Reagents: Ethyl (E)-4-(4-nitrophenyl)-4-oxo-2-butenoate, author=A. F. Renaldo , author2=J. W. Labadie , author3=J. K. Stille , journal=Org. Synth., year=1989, volume=67, page=86, doi=10.15227/orgsyn.067.0086 and reagents, such as tributyltin hydride Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compound
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less importan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redistribution Reaction
In chemistry, redistribution usually refers to the exchange of anionic ligands bonded to metal and metalloid centers. The conversion does not involve redox Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ..., in contrast to disproportionation reactions. Some useful redistribution reactions are conducted at higher temperatures; upon cooling the mixture, the product mixture is kinetically frozen and the individual products can be separated. In cases where redistribution is rapid at mild temperatures, the reaction is less useful synthetically but still important mechanistically. Examples Redistribution reactions are exhibited by methylboranes. Thus monomethyldiborane rapidly converts at room temperature to diborane and trimethylborane:. The authors refer to redistributions as "dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stannic Chloride
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound of tin and chlorine with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other tin compounds. It was first discovered by Andreas Libavius (1550–1616) and was known as ''spiritus fumans libavii''. Preparation It is prepared from reaction of chlorine gas with tin at : : Structure Anhydrous tin(IV) chloride solidifies at −33 °C to give monoclinic crystals with the P21/c space group. It is isostructural with SnBr4. The molecules adopt near-perfect tetrahedral symmetry with average Sn–Cl distances of 227.9(3) pm. Reactions Tin(IV) chloride is well known as a Lewis acid. Thus it forms hydrates. The pentahydrate SnCl4·5H2O was formerly known as butter of tin. These hydrates consist of ''cis''- nCl4(H2O)2molecules together with varying amounts of water of crystallization. The additional water molecules l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributyltin Hydride
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is used as a source of hydrogen atoms in organic synthesis. Synthesis and characterization The compound is produced by reduction of tributyltin oxide with polymethylhydrosiloxane: : 2 " eSi(H)Osub>n" + (Bu3Sn)2O → " eSi(OH)Osub>n" + 2 Bu3SnH It can also be synthesized by a reduction of tributyltin chloride with lithium aluminium hydride. The hydride is a distillable liquid that is mildly sensitive to air, decomposing to (Bu3Sn)2O. Its IR spectrum exhibits a strong band at 1814 cm−1 for ''ν''Sn−H. Applications It is a specialized reagent in organic synthesis. Combined with azobisisobutyronitrile (AIBN) or by irradiation with light, tributyltin hydride converts organic halides (and related groups) to the corresponding hydrocarbon. This process occurs via a radical chain mechanism involving the radical Bu3S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pronunciation of the word "chloride" is . Chloride salts such as sodium chloride are often soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Other examples of ionic chlorides include potassium chloride (), calcium chloride (), and ammonium chloride (). Examples of covalent chlorides include methyl chloride (), carbon tetrachloride (), sulfuryl chloride (), and monochloramine (). Electronic properties A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Halides
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are Ionic compound, ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride. File:NaCl polyhedra.svg, Sodium chloride crystal structure File:Uranium-hexafluoride-unit-cell-3D-balls.png, Discrete UF6 molecules File:Alpha-palladium(II)-chloride-xtal-3D-balls.png, Infinite chains of one form of palladium chloride Preparation The halogens can all react with metals to form metal halides according to the following equation: :2M + nX2 → 2MXn where M is the metal, X is the halogen, and MXn is the metal halide. In practice, this type of reaction may be very exothermic, hence impractical as a preparative technique. Additionally, many transition metals can adopt multiple oxidation states, which complicates matters. As the halogens are strong oxidizers, direct combination of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compounds
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin(IV) Compounds
Tin is a chemical element; it has chemical symbol, symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the so-called "tin cry", as a result of crystal twinning, twinning in tin crystals. Tin is a post-transition metal in Carbon group, group 14 of the periodic table of elements. It is obtained chiefly from the mineral cassiterite, which contains tin(IV) oxide, stannic oxide, . Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundance of the chemical elements, abundant element on Earth, making up 0.00022% of its crust, and with 10 stable isotopes, it has the largest number of stable isotopes in the periodic table, due to its magic number (physics), magic number of protons. It has two main allotrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |