
Corrosion is a
natural process that converts a refined metal into a more chemically stable
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
. It is the gradual deterioration of materials (usually a
metal) by chemical or electrochemical reaction with their environment.
Corrosion engineering is the field dedicated to controlling and preventing corrosion.
In the most common use of the word, this means
electrochemical oxidation of metal in reaction with an
oxidant such as
oxygen, hydrogen or hydroxide.
Rusting, the formation of
iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces
oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
(s) or
salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as
ceramics or
polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases.
Many structural
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s corrode merely from exposure to moisture in air, but the process can be strongly affected by exposure to certain substances. Corrosion can be concentrated locally to form a
pit
Pit or PIT may refer to:
Structure
* Ball pit, a recreation structure
* Casino pit, the part of a casino which holds gaming tables
* Trapping pit, pits used for hunting
* Pit (motor racing), an area of a racetrack where pit stops are conducted
* ...
or crack, or it can extend across a wide area more or less uniformly corroding the surface. Because corrosion is a diffusion-controlled process, it occurs on exposed surfaces. As a result, methods to reduce the activity of the exposed surface, such as
passivation and
chromate conversion
Chromate conversion coating or alodine coating is a type of conversion coating used to passivate steel, aluminium, zinc, cadmium, copper, silver, titanium, magnesium, and tin alloys. The coating serves as a corrosion inhibitor, as ...
, can increase a material's corrosion resistance. However, some corrosion mechanisms are less visible and less predictable.
The chemistry of corrosion is complex; it can be considered an
electrochemical phenomenon. During corrosion at a particular spot on the surface of an object made of iron,
oxidation takes place and that spot behaves as an
anode. The
electrons released at this anodic spot move through the
metal and go to another spot on the metal and
reduce oxygen at that spot in presence of H
+ (which is believed to be available from
carbonic acid () formed due to dissolution of
carbon dioxide from air into water in moist air condition of atmosphere.
Hydrogen ion in water may also be available due to dissolution of other acidic oxides from the atmosphere). This spot behaves as a
cathode.
Galvanic corrosion

Galvanic corrosion occurs when two different metals have physical or electrical contact with each other and are immersed in a common
electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
, or when the same metal is exposed to electrolyte with different concentrations. In a
galvanic couple
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
, the more active metal (the anode) corrodes at an accelerated rate and the more
noble metal (the cathode) corrodes at a slower rate. When immersed separately, each metal corrodes at its own rate. What type of metal(s) to use is readily determined by following the
galvanic series. For example, zinc is often used as a sacrificial anode for steel structures. Galvanic corrosion is of major interest to the marine industry and also anywhere water (containing salts) contacts pipes or metal structures.
Factors such as relative size of
anode, types of metal, and operating conditions (
temperature,
humidity,
salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
, etc.) affect galvanic corrosion. The surface area ratio of the anode and
cathode directly affects the corrosion rates of the materials. Galvanic corrosion is often prevented by the use of
sacrificial anode
A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged metal structures from corrosion.
They are made from a metal alloy with a more "active" voltage (more n ...
s.
Galvanic series
In any given environment (one standard medium is aerated, room-temperature
seawater), one metal will be either more ''noble'' or more ''active'' than others, based on how strongly its ions are bound to the surface. Two metals in electrical contact share the same electrons, so that the "tug-of-war" at each surface is analogous to competition for free electrons between the two materials. Using the electrolyte as a host for the flow of ions in the same direction, the noble metal will take electrons from the active one. The resulting mass flow or electric current can be measured to establish a hierarchy of materials in the medium of interest. This hierarchy is called a ''galvanic series'' and is useful in predicting and understanding corrosion.
Corrosion removal
Often it is possible to chemically remove the products of corrosion. For example,
phosphoric acid in the form of ''naval jelly'' is often applied to ferrous tools or surfaces to remove rust. Corrosion removal should not be confused with
electropolishing, which removes some layers of the underlying metal to make a smooth surface. For example, phosphoric acid may also be used to electropolish copper but it does this by removing copper, not the products of copper corrosion.
Resistance to corrosion
Some metals are more intrinsically resistant to corrosion than others (for some examples, see
galvanic series). There are various ways of
protecting metals from corrosion (oxidation) including painting,
hot-dip galvanization,
cathodic protection, and combinations of these.
Intrinsic chemistry

The materials most resistant to corrosion are those for which corrosion is
thermodynamically unfavorable. Any corrosion products of
gold or
platinum tend to decompose spontaneously into pure metal, which is why these elements can be found in metallic form on Earth and have long been valued. More common "base" metals can only be protected by more temporary means.
Some metals have naturally slow
reaction kinetics, even though their corrosion is thermodynamically favorable. These include such metals as
zinc,
magnesium, and
cadmium. While corrosion of these metals is continuous and ongoing, it happens at an acceptably slow rate. An extreme example is
graphite, which releases large amounts of energy upon
oxidation, but has such slow kinetics that it is effectively immune to electrochemical corrosion under normal conditions.
Passivation
Passivation refers to the spontaneous formation of an ultrathin film of corrosion products, known as a passive film, on the metal's surface that act as a barrier to further oxidation. The chemical composition and microstructure of a passive film are different from the underlying metal. Typical passive film thickness on aluminium, stainless steels, and alloys is within 10 nanometers. The passive film is different from oxide layers that are formed upon heating and are in the micrometer thickness range – the passive film recovers if removed or damaged whereas the oxide layer does not. Passivation in natural environments such as air, water and soil at moderate
pH is seen in such materials as
aluminium
Aluminium (aluminum in AmE, American and CanE, Canadian English) is a chemical element with the Symbol (chemistry), symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately o ...
,
stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
,
titanium, and
silicon.
Passivation is primarily determined by metallurgical and environmental factors. The effect of pH is summarized using
Pourbaix diagrams, but many other factors are influential. Some conditions that inhibit passivation include high pH for aluminium and zinc, low pH or the presence of
chloride ions for stainless steel, high temperature for titanium (in which case the oxide dissolves into the metal, rather than the electrolyte) and
fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typ ...
ions for silicon. On the other hand, unusual conditions may result in passivation of materials that are normally unprotected, as the alkaline environment of
concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most ...
does for
steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
rebar
Rebar (short for reinforcing bar), known when massed as reinforcing steel or reinforcement steel, is a steel bar used as a Tension (physics), tension device in reinforced concrete and reinforced masonry structures to strengthen and aid the concr ...
. Exposure to a liquid metal such as
mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
or hot
solder can often circumvent passivation mechanisms.
Corrosion in passivated materials
Passivation is extremely useful in mitigating corrosion damage, however even a high-quality alloy will corrode if its ability to form a passivating film is hindered. Proper selection of the right grade of material for the specific environment is important for the long-lasting performance of this group of materials. If breakdown occurs in the passive film due to chemical or mechanical factors, the resulting major modes of corrosion may include
pitting corrosion,
crevice corrosion, and
stress corrosion cracking
Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC ...
.
Pitting corrosion

Certain conditions, such as low concentrations of oxygen or high concentrations of species such as chloride which compete as
anions, can interfere with a given alloy's ability to re-form a passivating film. In the worst case, almost all of the surface will remain protected, but tiny local fluctuations will degrade the oxide film in a few critical points. Corrosion at these points will be greatly amplified, and can cause ''corrosion pits'' of several types, depending upon conditions. While the corrosion pits only
nucleate under fairly extreme circumstances, they can continue to grow even when conditions return to normal, since the interior of a pit is naturally deprived of oxygen and locally the pH decreases to very low values and the corrosion rate increases due to an autocatalytic process. In extreme cases, the sharp tips of extremely long and narrow corrosion pits can cause
stress concentration
In solid mechanics, a stress concentration (also called a stress raiser or a stress riser) is a location in an object where the stress is significantly greater than the surrounding region. Stress concentrations occur when there are irregularitie ...
to the point that otherwise tough alloys can shatter; a thin film pierced by an invisibly small hole can hide a thumb sized pit from view. These problems are especially dangerous because they are difficult to detect before a part or structure
fails. Pitting remains among the most common and damaging forms of corrosion in passivated alloys, but it can be prevented by control of the alloy's environment.
Pitting results when a small hole, or cavity, forms in the metal, usually as a result of de-passivation of a small area. This area becomes anodic, while part of the remaining metal becomes cathodic, producing a localized galvanic reaction. The deterioration of this small area penetrates the metal and can lead to failure. This form of corrosion is often difficult to detect due to the fact that it is usually relatively small and may be covered and hidden by corrosion-produced compounds.
Weld decay and knifeline attack


Stainless steel can pose special corrosion challenges, since its passivating behavior relies on the presence of a major alloying component (
chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
, at least 11.5%). Because of the elevated temperatures of
welding and heat treatment,
chromium carbides can form in the
grain boundaries of stainless alloys. This chemical reaction robs the material of chromium in the zone near the grain boundary, making those areas much less resistant to corrosion. This creates a
galvanic couple
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
with the well-protected alloy nearby, which leads to "weld decay" (corrosion of the grain boundaries in the heat affected zones) in highly corrosive environments. This process can seriously reduce the mechanical strength of welded joints over time.
A stainless steel is said to be "sensitized" if chromium carbides are formed in the microstructure. A typical microstructure of a normalized
type 304 stainless steel shows no signs of sensitization, while a heavily sensitized steel shows the presence of grain boundary precipitates. The dark lines in the sensitized microstructure are networks of chromium carbides formed along the grain boundaries.
Special alloys, either with low carbon content or with added carbon "
getters" such as titanium and
niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
(in types 321 and 347, respectively), can prevent this effect, but the latter require special heat treatment after welding to prevent the similar phenomenon of "knifeline attack". As its name implies, corrosion is limited to a very narrow zone adjacent to the weld, often only a few micrometers across, making it even less noticeable.
Crevice corrosion
 Crevice corrosion
Crevice corrosion is a localized form of corrosion occurring in confined spaces (crevices), to which the access of the working fluid from the environment is limited. Formation of a differential aeration cell leads to corrosion inside the crevices. Examples of crevices are gaps and contact areas between parts, under gaskets or seals, inside cracks and seams, spaces filled with deposits and under sludge piles.
Crevice corrosion is influenced by the crevice type (metal-metal, metal-non-metal), crevice geometry (size, surface finish), and metallurgical and environmental factors. The susceptibility to crevice corrosion can be evaluated with ASTM standard procedures. A critical crevice corrosion temperature is commonly used to rank a material's resistance to crevice corrosion.
Hydrogen grooving
In the
chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., wit ...
industry, hydrogen grooving is the corrosion of piping by grooves created by the interaction of a corrosive agent, corroded pipe constituents, and
hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
gas bubbles. For example, when
sulfuric acid () flows through
steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
pipes, the
iron in the steel reacts with the
acid to form a
passivation coating of
iron sulfate () and hydrogen gas (). The iron sulfate coating will protect the steel from further reaction; however, if hydrogen bubbles contact this coating, it will be removed. Thus, a groove will be formed by a travelling bubble, exposing more steel to the acid: a
vicious cycle. The grooving is exacerbated by the tendency of subsequent bubbles to follow the same path.
High-temperature corrosion
High-temperature corrosion
High-temperature corrosion is a mechanism of corrosion that takes place when gas turbines, diesel engines, furnaces or other machinery come in contact with hot gas containing certain contaminants. Fuel sometimes contains vanadium compounds or su ...
is chemical deterioration of a material (typically a metal) as a result of heating. This non-galvanic form of corrosion can occur when a metal is subjected to a hot atmosphere containing oxygen, sulfur, or other compounds capable of oxidizing (or assisting the oxidation of) the material concerned. For example, materials used in aerospace, power generation and even in car engines have to resist sustained periods at high temperature in which they may be exposed to an atmosphere containing potentially highly corrosive products of combustion.
The products of high-temperature corrosion can potentially be turned to the advantage of the engineer. The formation of oxides on stainless steels, for example, can provide a protective layer preventing further atmospheric attack, allowing for a material to be used for sustained periods at both room and high temperatures in hostile conditions. Such high-temperature corrosion products, in the form of
compacted oxide layer glaze
Compacted oxide layer glaze describes the often shiny, wear-protective layer of oxide formed when two Metal, metals (or a metal and ceramic) are slid against each other at high temperature in an oxygen-containing atmosphere. The layer forms on eith ...
s, prevent or reduce wear during high-temperature sliding contact of metallic (or metallic and ceramic) surfaces.
Thermal oxidation
In microfabrication, thermal oxidation is a way to produce a thin layer of oxide (usually silicon dioxide) on the surface of a wafer. The technique forces an oxidizing agent to diffuse into the wafer at high temperature and react with it. The ...
is also commonly used as a route towards the obtainment of controlled oxide nanostructures, including
nanowires and thin films.
Microbial corrosion
Microbial corrosion, or commonly known as microbiologically influenced corrosion (MIC), is a corrosion caused or promoted by
microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s, usually
chemoautotrophs. It can apply to both metallic and non-metallic materials, in the presence or absence of oxygen.
Sulfate-reducing bacteria are active in the absence of oxygen (anaerobic); they produce
hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
, causing
sulfide stress cracking. In the presence of oxygen (aerobic), some bacteria may directly oxidize iron to iron oxides and hydroxides, other bacteria oxidize sulfur and produce sulfuric acid causing
biogenic sulfide corrosion.
Concentration cells can form in the deposits of corrosion products, leading to localized corrosion.
Accelerated low-water corrosion (ALWC) is a particularly aggressive form of MIC that affects steel piles in seawater near the low water tide mark. It is characterized by an orange sludge, which smells of hydrogen sulfide when treated with acid. Corrosion rates can be very high and design corrosion allowances can soon be exceeded leading to premature failure of the steel pile. Piles that have been coated and have cathodic protection installed at the time of construction are not susceptible to ALWC. For unprotected piles, sacrificial anodes can be installed locally to the affected areas to inhibit the corrosion or a complete retrofitted sacrificial anode system can be installed. Affected areas can also be treated using cathodic protection, using either sacrificial anodes or applying current to an inert anode to produce a calcareous deposit, which will help shield the metal from further attack.
Metal dusting
Metal dusting Metal dusting is "a catastrophic form of corrosion that occurs when susceptible materials are exposed to environments with high carbon activities." The corrosion manifests itself as a break-up of bulk metal to metal powder. The suspected mechanism i ...
is a catastrophic form of corrosion that occurs when susceptible materials are exposed to environments with high carbon activities, such as synthesis gas and other high-CO environments. The corrosion manifests itself as a break-up of bulk metal to metal powder. The suspected mechanism is firstly the deposition of a graphite layer on the surface of the metal, usually from
carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
(CO) in the vapor phase. This graphite layer is then thought to form metastable M
3C species (where M is the metal), which migrate away from the metal surface. However, in some regimes no M
3C species is observed indicating a direct transfer of metal atoms into the graphite layer.
Protection from corrosion

Various treatments are used to slow corrosion damage to metallic objects which are exposed to the weather, salt water, acids, or other hostile environments. Some unprotected metallic alloys are extremely vulnerable to corrosion, such as those used in
neodymium magnet
A Nickel-plated neodymium magnet on a bracket from a thumb">Nickel-plated neodymium magnet cubes
Left: high-resolution transmission electron microscopy image of Nd2Fe14B; right: unit_cell.html" ;"title="crystal structure with unit cell">crysta ...
s, which can
spall or crumble into powder even in dry, temperature-stable indoor environments unless properly treated to discourage corrosion.
Surface treatments
When surface treatments are used to deter corrosion, great care must be taken to ensure complete coverage, without gaps, cracks, or pinhole defects. Small defects can act as an "
Achilles' heel", allowing corrosion to penetrate the interior and causing extensive damage even while the outer protective layer remains apparently intact for a period of time.
Applied coatings
 Plating
Plating,
paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
ing, and the application of
enamel are the most common
anti-corrosion treatments. They work by providing a barrier of corrosion-resistant material between the damaging environment and the structural material. Aside from cosmetic and manufacturing issues, there may be tradeoffs in mechanical flexibility versus resistance to abrasion and high temperature. Platings usually fail only in small sections, but if the plating is more noble than the substrate (for example, chromium on steel), a
galvanic couple
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
will cause any exposed area to corrode much more rapidly than an unplated surface would. For this reason, it is often wise to plate with active metal such as
zinc or
cadmium. If the zinc coating is not thick enough the surface soon becomes unsightly with rusting obvious. The design life is directly related to the metal coating thickness.

Painting either by roller or brush is more desirable for tight spaces; spray would be better for larger coating areas such as steel decks and waterfront applications. Flexible
polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethan ...
coatings, like Durabak-M26 for example, can provide an anti-corrosive seal with a highly durable slip resistant membrane. Painted coatings are relatively easy to apply and have fast drying times although temperature and humidity may cause dry times to vary.
Nowadays, organic coatings made using petroleum based polymer are being replaced with many renewable source based organic coatings. Among various vehicles or binders, polyurethanes are the most explored polymer in such a attempts.
Reactive coatings
If the environment is controlled (especially in recirculating systems),
corrosion inhibitors can often be added to it. These chemicals form an electrically insulating or chemically impermeable coating on exposed metal surfaces, to suppress electrochemical reactions. Such methods make the system less sensitive to scratches or defects in the coating, since extra inhibitors can be made available wherever metal becomes exposed. Chemicals that inhibit corrosion include some of the salts in
hard water (Roman water systems are famous for their
mineral deposits),
chromates,
phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
s,
polyaniline, other
conducting polymers
Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromole ...
and a wide range of specially designed chemicals that resemble
surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, fo ...
s (i.e. long-chain organic molecules with ionic end groups).
Anodization
 Aluminium alloy
Aluminium alloys often undergo a surface treatment. Electrochemical conditions in the bath are carefully adjusted so that uniform pores, several
nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, ...
s wide, appear in the metal's oxide film. These pores allow the oxide to grow much thicker than passivating conditions would allow. At the end of the treatment, the pores are allowed to seal, forming a harder-than-usual surface layer. If this coating is scratched, normal passivation processes take over to protect the damaged area.
Anodizing is very resilient to weathering and corrosion, so it is commonly used for building facades and other areas where the surface will come into regular contact with the elements. While being resilient, it must be cleaned frequently. If left without cleaning,
panel edge staining will naturally occur. Anodization is the process of converting an anode into cathode by bringing a more active anode in contact with it.
Biofilm coatings
A new form of protection has been developed by applying certain species of bacterial films to the surface of metals in highly corrosive environments. This process increases the corrosion resistance substantially. Alternatively, antimicrobial-producing
biofilms can be used to inhibit mild steel corrosion from
sulfate-reducing bacteria.
Controlled permeability formwork
Controlled permeability formwork (CPF) is a method of preventing the corrosion of
reinforcement by naturally enhancing the durability of the
cover during concrete placement. CPF has been used in environments to combat the effects of
carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids.
In inorganic ...
, chlorides,
frost
Frost is a thin layer of ice on a solid surface, which forms from water vapor in an above- freezing atmosphere coming in contact with a solid surface whose temperature is below freezing, and resulting in a phase change from water vapor (a g ...
and abrasion.
Cathodic protection
Cathodic protection (CP) is a technique to control the corrosion of a metal surface by making it the cathode of an
electrochemical cell. Cathodic protection systems are most commonly used to protect steel
pipelines and tanks; steel pier
piles
Hemorrhoids (or haemorrhoids), also known as piles, are vascular structures in the anal canal. In their normal state, they are cushions that help with stool control. They become a disease when swollen or inflamed; the unqualified term ''hemo ...
, ships, and offshore
oil platforms.
Sacrificial anode protection

For effective CP, the potential of the steel surface is polarized (pushed) more negative until the metal surface has a uniform potential. With a uniform potential, the driving force for the corrosion reaction is halted. For galvanic CP systems, the anode material corrodes under the influence of the steel, and eventually it must be replaced. The
polarization
Polarization or polarisation may refer to:
Mathematics
*Polarization of an Abelian variety, in the mathematics of complex manifolds
*Polarization of an algebraic form, a technique for expressing a homogeneous polynomial in a simpler fashion by ...
is caused by the current flow from the anode to the cathode, driven by the difference in
electrode potential
In electrochemistry, electrode potential is the electromotive force of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrod ...
between the anode and the cathode. The most common sacrificial anode materials are aluminum, zinc, magnesium and related alloys. Aluminum has the highest capacity, and magnesium has the highest driving voltage and is thus used where resistance is higher. Zinc is general purpose and the basis for galvanizing.
A number of problems are associated with sacrificial anodes. Among these, from an environmental perspective, is the release of zinc, magnesium, aluminum and heavy metals such as
cadmium into the environment including seawater. From a working perspective, sacrificial anodes systems are considered to be less precise than modern cathodic protection systems such as Impressed Current Cathodic Protection (ICCP) systems. Their ability to provide requisite protection has to be checked regularly by means of underwater inspection by divers. Furthermore, as they have a finite lifespan, sacrificial anodes need to be replaced regularly over time.
Impressed current cathodic protection
For larger structures, galvanic anodes cannot economically deliver enough current to provide complete protection.
Impressed current cathodic protection (ICCP) systems use anodes connected to a
DC power source (such as a
cathodic protection rectifier
Cathodic protection (CP; ) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded "sacrific ...
). Anodes for ICCP systems are tubular and solid rod shapes of various specialized materials. These include high silicon
cast iron
Cast iron is a class of iron– carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impu ...
, graphite, mixed metal oxide or platinum coated titanium or niobium coated rod and wires.
Anodic protection
Anodic protection impresses anodic current on the structure to be protected (opposite to the cathodic protection). It is appropriate for metals that exhibit passivity (e.g. stainless steel) and suitably small passive current over a wide range of potentials. It is used in aggressive environments, such as solutions of sulfuric acid. Anodic protection is an electrochemical method of corrosion protection by keeping metal in passive state
Rate of corrosion

The formation of an oxide layer is described by the
Deal–Grove model, which is used to predict and control oxide layer formation in diverse situations. A simple test for measuring corrosion is the weight loss method. The method involves exposing a clean weighed piece of the metal or alloy to the corrosive environment for a specified time followed by cleaning to remove corrosion products and weighing the piece to determine the loss of weight. The rate of corrosion (R) is calculated as
:
where ''k'' is a constant,
''W'' is the weight loss of the metal in time ''t'', ''A'' is the surface area of the metal exposed, and ''ρ'' is the density of the metal (in g/cm
3).
Other common expressions for the corrosion rate is penetration depth and change of mechanical properties.
Economic impact

In 2002, the US
Federal Highway Administration
The Federal Highway Administration (FHWA) is a division of the United States Department of Transportation that specializes in highway transportation. The agency's major activities are grouped into two programs, the Federal-aid Highway Program ...
released a study titled "Corrosion Costs and Preventive Strategies in the United States" on the direct costs associated with metallic corrosion in the US industry. In 1998, the total annual direct cost of corrosion in the U.S. was ca. $276 billion (ca. 3.2% of the US
gross domestic product
Gross domestic product (GDP) is a money, monetary Measurement in economics, measure of the market value of all the final goods and services produced and sold (not resold) in a specific time period by countries. Due to its complex and subjec ...
). Broken down into five specific industries, the economic losses are $22.6 billion in infrastructure; $17.6 billion in production and manufacturing; $29.7 billion in transportation; $20.1 billion in government; and $47.9 billion in utilities.
Rust is one of the most common causes of bridge accidents. As rust has a much higher volume than the originating mass of iron, its build-up can also cause failure by forcing apart adjacent parts. It was the cause of the collapse of the
Mianus River Bridge
The Mianus River Bridge is a span that carries Interstate 95 (Connecticut Turnpike) over the Mianus River, between Cos Cob and Riverside, Connecticut. It is the second bridge on the site. The original bridge collapsed in 1983, killing three mot ...
in 1983, when the bearings rusted internally and pushed one corner of the road slab off its support. Three drivers on the roadway at the time died as the slab fell into the river below. The following
NTSB investigation showed that a drain in the road had been blocked for road re-surfacing, and had not been unblocked; as a result, runoff water penetrated the support hangers. Rust was also an important factor in the
Silver Bridge disaster of 1967 in
West Virginia
West Virginia is a state in the Appalachian, Mid-Atlantic and Southeastern regions of the United States.The Census Bureau and the Association of American Geographers classify West Virginia as part of the Southern United States while the ...
, when a steel
suspension bridge collapsed within a minute, killing 46 drivers and passengers on the bridge at the time.
Similarly, corrosion of concrete-covered steel and iron can cause the concrete to
spall, creating severe structural problems. It is one of the most common failure modes of
reinforced concrete bridge
A bridge is a structure built to span a physical obstacle (such as a body of water, valley, road, or rail) without blocking the way underneath. It is constructed for the purpose of providing passage over the obstacle, which is usually somethi ...
s. Measuring instruments based on the half-cell potential can detect the potential corrosion spots before total failure of the concrete structure is reached.
Until 20–30 years ago, galvanized steel pipe was used extensively in the potable water systems for single and multi-family residents as well as commercial and public construction. Today, these systems have long ago consumed the protective zinc and are corroding internally resulting in poor water quality and pipe failures. The economic impact on homeowners, condo dwellers, and the public infrastructure is estimated at 22 billion dollars as the insurance industry braces for a wave of claims due to pipe failures.
Corrosion in nonmetals
Most
ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelai ...
materials are almost entirely immune to corrosion. The strong
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
s that hold them together leave very little free chemical energy in the structure; they can be thought of as already corroded. When corrosion does occur, it is almost always a simple dissolution of the material or chemical reaction, rather than an electrochemical process. A common example of corrosion protection in ceramics is the
lime added to soda-lime
glass
Glass is a non-Crystallinity, crystalline, often transparency and translucency, transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most ...
to reduce its solubility in water; though it is not nearly as soluble as pure
sodium silicate, normal glass does form sub-microscopic flaws when exposed to moisture. Due to its
brittleness, such flaws cause a dramatic reduction in the strength of a glass object during its first few hours at room temperature.
Corrosion of polymers
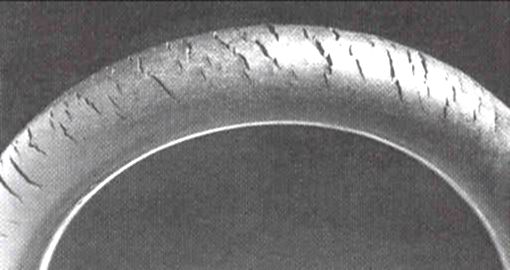 Polymer degradation
Polymer degradation involves several complex and often poorly understood physiochemical processes. These are strikingly different from the other processes discussed here, and so the term "corrosion" is only applied to them in a loose sense of the word. Because of their large molecular weight, very little
entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
can be gained by mixing a given mass of polymer with another substance, making them generally quite difficult to dissolve. While dissolution is a problem in some polymer applications, it is relatively simple to design against.
A more common and related problem is "swelling", where small molecules infiltrate the structure, reducing strength and stiffness and causing a volume change. Conversely, many polymers (notably flexible
vinyl) are intentionally swelled with
plasticizer
A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture.
Plasticiz ...
s, which can be leached out of the structure, causing brittleness or other undesirable changes.
The most common form of degradation, however, is a decrease in polymer chain length. Mechanisms which break polymer chains are familiar to biologists because of their effect on
DNA:
ionizing radiation (most commonly
ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiati ...
light),
free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
s, and
oxidizers such as oxygen,
ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
, and
chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is ...
.
Ozone cracking is a well-known problem affecting
natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, a ...
for example. Plastic additives can slow these process very effectively, and can be as simple as a UV-absorbing
pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic comp ...
(e.g.
titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insoluble ...
or
carbon black
Carbon black (subtypes are acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal and coal tar, vegetable matter, or petroleum products, including fuel oil, fluid ...
).
Plastic shopping bags often do not include these additives so that they break down more easily as ultrafine particles of
litter
Litter consists of waste products that have been discarded incorrectly, without consent, at an unsuitable location. Litter can also be used as a verb; to litter means to drop and leave objects, often man-made, such as aluminum cans, paper cups ...
.
Corrosion of glass

Glass is characterized by a high degree of corrosion-resistance. Because of its high water-resistance it is often used as primary packaging material in the pharma industry since most medicines are preserved in a watery solution. Besides its water-resistance, glass is also robust when exposed to certain chemically aggressive liquids or gases.
Glass disease is the corrosion of silicate glasses in
aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would ...
s. It is governed by two mechanisms:
diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
-
controlled leaching (ion exchange) and hydrolytic dissolution of the glass network. Both mechanisms strongly depend on the pH of contacting solution: the rate of ion exchange decreases with pH as 10
−0.5pH whereas the rate of hydrolytic dissolution increases with pH as 10
0.5pH.
Mathematically, corrosion rates of glasses are characterized by normalized corrosion rates of elements NR
''i'' (g/cm
2·d) which are determined as the ratio of total amount of released species into the water M
''i'' (g) to the water-contacting surface area S (cm
2), time of contact t (days) and weight fraction content of the element in the glass f
''i'':
:
.
The overall corrosion rate is a sum of contributions from both mechanisms (leaching + dissolution) NR
''i''=NR''x''
''i''+NR''h''.
Diffusion-controlled leaching (ion exchange) is characteristic of the initial phase of corrosion and involves replacement of alkali ions in the glass by a hydronium (H
3O
+) ion from the solution. It causes an ion-selective depletion of near surface layers of glasses and gives an inverse square root dependence of corrosion rate with exposure time. The diffusion-controlled normalized leaching rate of cations from glasses (g/cm
2·d) is given by:
:
,
where t is time, ''D''
''i'' is the i-th cation effective diffusion coefficient (cm
2/d), which depends on pH of contacting water as ''D''
''i'' = D
''i''0·10
–pH, and ''ρ'' is the density of the glass (g/cm
3).
Glass network dissolution is characteristic of the later phases of corrosion and causes a congruent release of ions into the water solution at a time-independent rate in dilute solutions (g/cm
2·d):
:
,
where r
h is the stationary
hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
(dissolution) rate of the glass (cm/d).
In closed systems the consumption of protons from the aqueous phase increases the pH and causes a fast transition to hydrolysis. However, a further saturation of solution with silica impedes hydrolysis and causes the glass to return to an ion-exchange, e.g. diffusion-controlled regime of corrosion.
In typical natural conditions normalized corrosion rates of silicate glasses are very low and are of the order of 10
−7–10
−5 g/(cm
2·d). The very high durability of silicate glasses in water makes them suitable for hazardous and nuclear waste immobilisation.
Glass corrosion tests
There exist numerous standardized procedures for measuring the corrosion (also called chemical durability) of glasses in neutral, basic, and acidic environments, under simulated environmental conditions, in simulated body fluid, at high temperature and pressure, and under other conditions.
The standard procedure ISO 719
International Organization for Standardization, Procedure 719 (1985)
Iso.org (2011-01-21). Retrieved on 2012-07-15. describes a test of the extraction of water-soluble basic compounds under neutral conditions: 2 g of glass, particle size 300–500 μm, is kept for 60 min in 50 mL de-ionized water of grade 2 at 98 °C; 25 mL of the obtained solution is titrated against 0.01 mol/L HCl solution. The volume of HCl required for neutralization is classified according to the table below.
The standardized test ISO 719 is not suitable for glasses with poor or not extractable alkaline components, but which are still attacked by water, e.g. quartz glass, B2O3 glass or P2O5 glass.
Usual glasses are differentiated into the following classes:
Hydrolytic class 1 (Type I):
This class, which is also called neutral glass, includes borosilicate glass
Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion (≈3 × 10−6 K−1 at 20 °C), m ...
es (e.g. Duran, Pyrex, Fiolax).
Glass of this class contains essential quantities of boron oxides, aluminium oxides and alkaline earth oxides. Through its composition neutral glass has a high resistance against temperature shocks and the highest hydrolytic resistance. Against acid and neutral solutions it shows high chemical resistance, because of its poor alkali content against alkaline solutions.
Hydrolytic class 2 (Type II):
This class usually contains sodium silicate glasses with a high hydrolytic resistance through surface finishing. Sodium silicate glass is a silicate glass, which contains alkali- and alkaline earth oxide and primarily sodium oxide
Sodium oxide is a chemical compound with the formula Na2 O. It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glass ...
and Calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic ...
.
Hydrolytic class 3 (Type III):
Glass of the 3rd hydrolytic class usually contains sodium silicate glasses and has a mean hydrolytic resistance, which is two times poorer than of type 1 glasses.
Acid class DIN 12116 and alkali class DIN 52322 (ISO 695) are to be distinguished from the hydrolytic class DIN 12111 (ISO 719).
See also
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
References
Further reading
*
{{Authority control
Glass chemistry
Metallurgy
 Galvanic corrosion occurs when two different metals have physical or electrical contact with each other and are immersed in a common
Galvanic corrosion occurs when two different metals have physical or electrical contact with each other and are immersed in a common  The materials most resistant to corrosion are those for which corrosion is thermodynamically unfavorable. Any corrosion products of gold or platinum tend to decompose spontaneously into pure metal, which is why these elements can be found in metallic form on Earth and have long been valued. More common "base" metals can only be protected by more temporary means.
Some metals have naturally slow reaction kinetics, even though their corrosion is thermodynamically favorable. These include such metals as zinc, magnesium, and cadmium. While corrosion of these metals is continuous and ongoing, it happens at an acceptably slow rate. An extreme example is graphite, which releases large amounts of energy upon oxidation, but has such slow kinetics that it is effectively immune to electrochemical corrosion under normal conditions.
The materials most resistant to corrosion are those for which corrosion is thermodynamically unfavorable. Any corrosion products of gold or platinum tend to decompose spontaneously into pure metal, which is why these elements can be found in metallic form on Earth and have long been valued. More common "base" metals can only be protected by more temporary means.
Some metals have naturally slow reaction kinetics, even though their corrosion is thermodynamically favorable. These include such metals as zinc, magnesium, and cadmium. While corrosion of these metals is continuous and ongoing, it happens at an acceptably slow rate. An extreme example is graphite, which releases large amounts of energy upon oxidation, but has such slow kinetics that it is effectively immune to electrochemical corrosion under normal conditions.
 Certain conditions, such as low concentrations of oxygen or high concentrations of species such as chloride which compete as anions, can interfere with a given alloy's ability to re-form a passivating film. In the worst case, almost all of the surface will remain protected, but tiny local fluctuations will degrade the oxide film in a few critical points. Corrosion at these points will be greatly amplified, and can cause ''corrosion pits'' of several types, depending upon conditions. While the corrosion pits only nucleate under fairly extreme circumstances, they can continue to grow even when conditions return to normal, since the interior of a pit is naturally deprived of oxygen and locally the pH decreases to very low values and the corrosion rate increases due to an autocatalytic process. In extreme cases, the sharp tips of extremely long and narrow corrosion pits can cause
Certain conditions, such as low concentrations of oxygen or high concentrations of species such as chloride which compete as anions, can interfere with a given alloy's ability to re-form a passivating film. In the worst case, almost all of the surface will remain protected, but tiny local fluctuations will degrade the oxide film in a few critical points. Corrosion at these points will be greatly amplified, and can cause ''corrosion pits'' of several types, depending upon conditions. While the corrosion pits only nucleate under fairly extreme circumstances, they can continue to grow even when conditions return to normal, since the interior of a pit is naturally deprived of oxygen and locally the pH decreases to very low values and the corrosion rate increases due to an autocatalytic process. In extreme cases, the sharp tips of extremely long and narrow corrosion pits can cause 
 Stainless steel can pose special corrosion challenges, since its passivating behavior relies on the presence of a major alloying component (
Stainless steel can pose special corrosion challenges, since its passivating behavior relies on the presence of a major alloying component ( Crevice corrosion is a localized form of corrosion occurring in confined spaces (crevices), to which the access of the working fluid from the environment is limited. Formation of a differential aeration cell leads to corrosion inside the crevices. Examples of crevices are gaps and contact areas between parts, under gaskets or seals, inside cracks and seams, spaces filled with deposits and under sludge piles.
Crevice corrosion is influenced by the crevice type (metal-metal, metal-non-metal), crevice geometry (size, surface finish), and metallurgical and environmental factors. The susceptibility to crevice corrosion can be evaluated with ASTM standard procedures. A critical crevice corrosion temperature is commonly used to rank a material's resistance to crevice corrosion.
Crevice corrosion is a localized form of corrosion occurring in confined spaces (crevices), to which the access of the working fluid from the environment is limited. Formation of a differential aeration cell leads to corrosion inside the crevices. Examples of crevices are gaps and contact areas between parts, under gaskets or seals, inside cracks and seams, spaces filled with deposits and under sludge piles.
Crevice corrosion is influenced by the crevice type (metal-metal, metal-non-metal), crevice geometry (size, surface finish), and metallurgical and environmental factors. The susceptibility to crevice corrosion can be evaluated with ASTM standard procedures. A critical crevice corrosion temperature is commonly used to rank a material's resistance to crevice corrosion.
 Various treatments are used to slow corrosion damage to metallic objects which are exposed to the weather, salt water, acids, or other hostile environments. Some unprotected metallic alloys are extremely vulnerable to corrosion, such as those used in
Various treatments are used to slow corrosion damage to metallic objects which are exposed to the weather, salt water, acids, or other hostile environments. Some unprotected metallic alloys are extremely vulnerable to corrosion, such as those used in  Plating,
Plating,  Painting either by roller or brush is more desirable for tight spaces; spray would be better for larger coating areas such as steel decks and waterfront applications. Flexible
Painting either by roller or brush is more desirable for tight spaces; spray would be better for larger coating areas such as steel decks and waterfront applications. Flexible  Aluminium alloys often undergo a surface treatment. Electrochemical conditions in the bath are carefully adjusted so that uniform pores, several
Aluminium alloys often undergo a surface treatment. Electrochemical conditions in the bath are carefully adjusted so that uniform pores, several  For effective CP, the potential of the steel surface is polarized (pushed) more negative until the metal surface has a uniform potential. With a uniform potential, the driving force for the corrosion reaction is halted. For galvanic CP systems, the anode material corrodes under the influence of the steel, and eventually it must be replaced. The
For effective CP, the potential of the steel surface is polarized (pushed) more negative until the metal surface has a uniform potential. With a uniform potential, the driving force for the corrosion reaction is halted. For galvanic CP systems, the anode material corrodes under the influence of the steel, and eventually it must be replaced. The  The formation of an oxide layer is described by the Deal–Grove model, which is used to predict and control oxide layer formation in diverse situations. A simple test for measuring corrosion is the weight loss method. The method involves exposing a clean weighed piece of the metal or alloy to the corrosive environment for a specified time followed by cleaning to remove corrosion products and weighing the piece to determine the loss of weight. The rate of corrosion (R) is calculated as
:
where ''k'' is a constant,
''W'' is the weight loss of the metal in time ''t'', ''A'' is the surface area of the metal exposed, and ''ρ'' is the density of the metal (in g/cm3).
Other common expressions for the corrosion rate is penetration depth and change of mechanical properties.
The formation of an oxide layer is described by the Deal–Grove model, which is used to predict and control oxide layer formation in diverse situations. A simple test for measuring corrosion is the weight loss method. The method involves exposing a clean weighed piece of the metal or alloy to the corrosive environment for a specified time followed by cleaning to remove corrosion products and weighing the piece to determine the loss of weight. The rate of corrosion (R) is calculated as
:
where ''k'' is a constant,
''W'' is the weight loss of the metal in time ''t'', ''A'' is the surface area of the metal exposed, and ''ρ'' is the density of the metal (in g/cm3).
Other common expressions for the corrosion rate is penetration depth and change of mechanical properties.
 In 2002, the US
In 2002, the US  Polymer degradation involves several complex and often poorly understood physiochemical processes. These are strikingly different from the other processes discussed here, and so the term "corrosion" is only applied to them in a loose sense of the word. Because of their large molecular weight, very little
Polymer degradation involves several complex and often poorly understood physiochemical processes. These are strikingly different from the other processes discussed here, and so the term "corrosion" is only applied to them in a loose sense of the word. Because of their large molecular weight, very little  Glass is characterized by a high degree of corrosion-resistance. Because of its high water-resistance it is often used as primary packaging material in the pharma industry since most medicines are preserved in a watery solution. Besides its water-resistance, glass is also robust when exposed to certain chemically aggressive liquids or gases.
Glass disease is the corrosion of silicate glasses in
Glass is characterized by a high degree of corrosion-resistance. Because of its high water-resistance it is often used as primary packaging material in the pharma industry since most medicines are preserved in a watery solution. Besides its water-resistance, glass is also robust when exposed to certain chemically aggressive liquids or gases.
Glass disease is the corrosion of silicate glasses in