|
Carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids. In inorganic chemistry and geology, carbonation is common. Metal hydroxides (MOH) and metal oxides (M'O) react with CO2 to give bicarbonates and carbonates: :MOH + CO2 → M(HCO3) :M'O + CO2 → M'CO3 Selected carbonations Carbonic anhydrase In mammalian physiology, transport of carbon dioxide to the lungs involves a carbonation reaction catalyzed by the enzyme carbonic anhydrase. In the absence of such catalysts, carbon dioxide cannot be expelled sufficient rate to support metabolic needs. The enzyme harbors a zinc aquo complex, which captures carbon dioxide to give a zinc bicarbonate: : Behavior of concrete In reinforced concrete, the chemical reaction between carbon dioxide in the air and calcium hydroxide and hydrated calcium silicate in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carboxylation
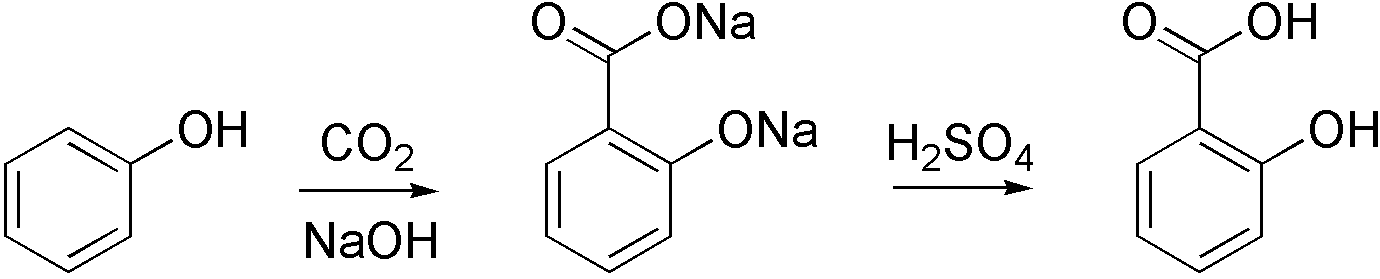
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates. Organic chemistry Carboxylation is a standard conversion in organic chemistry. Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids. Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid. : Many detailed procedures are d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Reinforced Concrete
Reinforced concrete, also called ferroconcrete or ferro-concrete, is a composite material in which concrete's relatively low tensile strength and ductility are compensated for by the inclusion of reinforcement having higher tensile strength or ductility. The reinforcement is usually, though not necessarily, steel reinforcing bars (known as rebar) and is usually embedded passively in the concrete before the concrete sets. However, post-tensioning is also employed as a technique to reinforce the concrete. In terms of volume used annually, it is one of the most common engineering materials. In corrosion engineering terms, when designed correctly, the alkalinity of the concrete protects the steel rebar from corrosion. Description Reinforcing schemes are generally designed to resist tensile stresses in particular regions of the concrete that might cause unacceptable cracking and/or structural failure. Modern reinforced concrete can contain varied reinforcing materials made o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
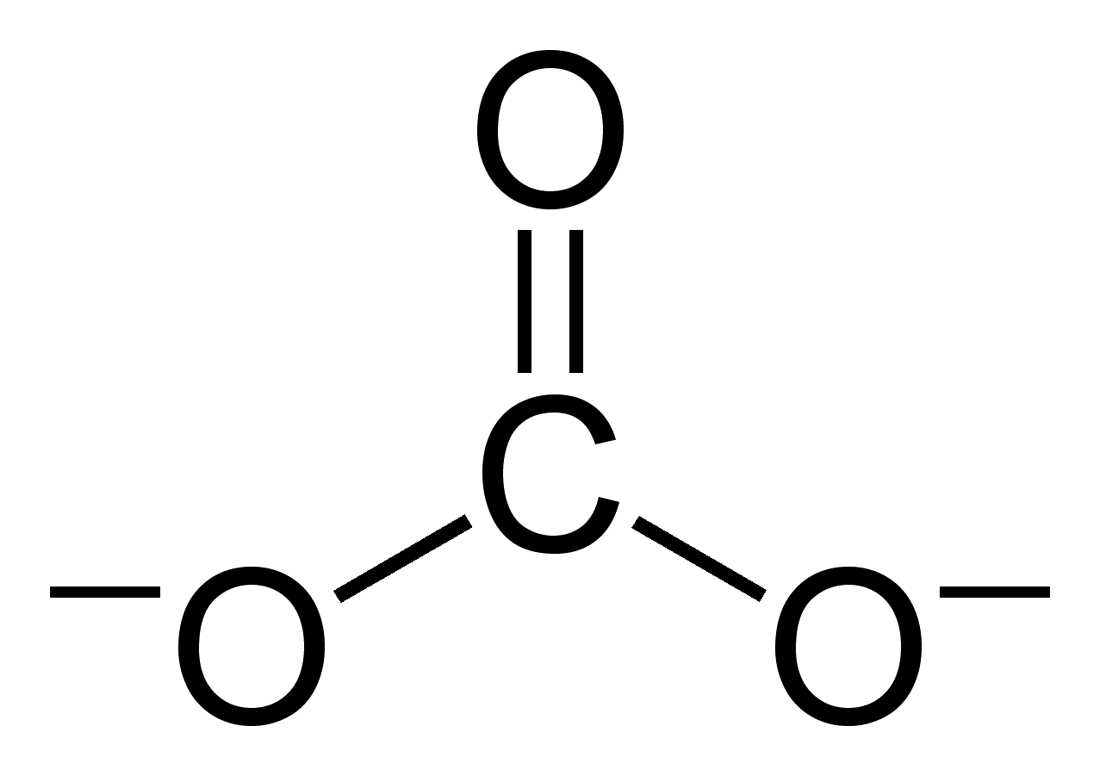
Carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group . The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, , the chief constituent of limestone (as well as the main component of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbonatation
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate: :Ca(OH)2CO2->CaCO3H_2O The process of forming a carbonate is sometimes referred to as "carbonation", although this term usually refers to the process of dissolving carbon dioxide in water. Concrete Carbonatation is a slow process that occurs in concrete where lime ( CaO, or Ca(OH)2( aq)) in the cement reacts with carbon dioxide (CO2) from the air and forms calcium carbonate. The water in the pores of Portland cement concrete is normally alkaline with a pH in the range of 12.5 to 13.5. This highly alkaline environment is one in which the steel rebar is passivated and is protected from corrosion. According to the Pourbaix diagram for iron, the metal is passive when the pH is above 9.5.{{cite web , url=http://www.corrosion-doctors.org/Thermo/ironE-pH.htm , title=Pourbaix diagram of iron , publisher=Corrosion-doctors.org , date= , accessdate=2009- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Geology
Geology (). is a branch of natural science concerned with the Earth and other astronomical objects, the rocks of which they are composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth sciences, including hydrology. It is integrated with Earth system science and planetary science. Geology describes the structure of the Earth on and beneath its surface and the processes that have shaped that structure. Geologists study the mineralogical composition of rocks in order to get insight into their history of formation. Geology determines the relative ages of rocks found at a given location; geochemistry (a branch of geology) determines their absolute ages. By combining various petrological, crystallographic, and paleontological tools, geologists are able to chronicle the geological history of the Earth as a whole. One aspect is to demonstrate the age of the Earth. Geology provides evidence for plate tectonics, the ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Inorganic Chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, pharmaceutical drug, medications, fuels, and agriculture. Occurrence Many inorganic compounds are found in nature as minerals. Soil may contain iron sulfide as pyrite or calcium sulfate as gypsum. Inorganic compounds are also found multitasking as biomolecules: as electrolytes (sodium chloride), in energy storage (Adenosine triphosphate, ATP) or in construction (the polyphosphate backbone in DNA). Bonding Inorganic compounds exhibit a range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Mole Fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the total amount of all constituents in a mixture, ''n''tot (also expressed in moles): :x_i = \frac It is denoted ''xi'' (lowercase Roman letter '' x''), sometimes (lowercase Greek letter chi). (For mixtures of gases, the letter ''y'' is recommended.) It is a dimensionless quantity with dimension of \mathsf/\mathsf and dimensionless unit of moles per mole (mol/mol or molmol−1) or simply 1; metric prefixes may also be used (e.g., nmol/mol for 10−9). When expressed in percent, it is known as the mole percent or molar percentage (unit symbol %, sometimes "mol%", equivalent to cmol/mol for 10−2). The mole fraction is called amount fraction by the International Union of Pure and Applied Chemistry (IUPAC) and amount-of-substance fractio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Henry's Law
In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is directly proportional at equilibrium to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulated by the English chemist William Henry, who studied the topic in the early 19th century. In simple words, it states that the partial pressure of a gas in the vapour phase is directly proportional to the mole fraction of a gas in solution. An example where Henry's law is at play is the depth-dependent dissolution of oxygen and nitrogen in the blood of underwater divers that changes during decompression, going to decompression sickness. An everyday example is carbonated soft drinks, which contain dissolved carbon dioxide. Before opening, the gas above the drink in its container is almost pure carbon dioxide, at a pressure higher than atmospheric pressure. After the bottle is opened, this gas escapes, moving the partial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the cellular metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. ''Urea'' is Neo-Latin, , , itself from Proto-Indo-European ''*h₂worsom''. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor base (chemistry), alkaline. The body uses it in many processes, most notably metabolic waste#Nitrogen wastes, nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Neutralization (chemistry)
In chemistry, neutralization or neutralisation (see American and British English spelling differences, spelling differences) is a chemical reaction in which acid and a base (chemistry), base react with an equivalent quantity of each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants. Meaning of "neutralization" In the context of a chemical reaction the term neutralization is used for a reaction between an acid and a base (chemistry), base or alkali. Historically, this reaction was represented as :acid + base (alkali) → salt + water : For example: :HCl + NaOH → NaCl + H2O The statement is still valid as long as it is understood that in an aqueous solution the substances involved are subject to dissociated, dissociation, which changes the ionization state of the substances. The arrow sign, →, is used because ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |