Copaxone on:
[Wikipedia]
[Google]
[Amazon]
Glatiramer acetate, sold under the brand name Copaxone among others, is an
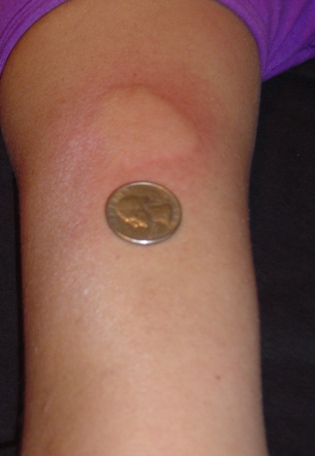 In the US, the prescription label includes a
In the US, the prescription label includes a
immunomodulator
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
medication used to treat multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease resulting in damage to myelinthe insulating covers of nerve cellsin the brain and spinal cord. As a demyelinating disease, MS disrupts the nervous system's ability to Action potential, transmit ...
. Glatiramer acetate is approved in the United States to reduce the frequency of relapses, but not for reducing the progression of disability. Observational studies
In fields such as epidemiology, social sciences, psychology and statistics, an observational study draws inferences from a sample to a population where the independent variable is not under the control of the researcher because of ethical conc ...
, but not randomized controlled trials, suggest that it may reduce progression of disability. While a conclusive diagnosis of multiple sclerosis requires a history of two or more episodes of symptoms and signs, glatiramer acetate is approved to treat a first episode anticipating a diagnosis. It is also used to treat relapsing-remitting multiple sclerosis. It is administered by subcutaneous injection
Subcutaneous administration is the insertion of medications beneath the skin either by injection or infusion.
A subcutaneous injection is administered as a bolus (medicine), bolus into the subcutis, the layer of skin directly below the dermis and ...
.
It is a mixture of random-sized peptides that are composed of the four amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
found in myelin basic protein
Myelin basic protein (MBP) is a protein important in the process of myelination of nerves in the nervous system. The myelin sheath is a multi-layered membrane, unique to the nervous system, that functions as an insulator to greatly increase the ve ...
, namely glutamic acid
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α- amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can ...
, lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group ( ...
, alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group sid ...
, and tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
. Myelin basic protein is the antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
...
in the myelin sheaths of the neurons that stimulates an autoimmune
In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an " autoimmune disease" ...
reaction in people with MS, so the peptide may work as a decoy for the attacking immune cells.
Glatiramer acetate was originally discovered at the Weizmann Institute of Science
The Weizmann Institute of Science ( ''Machon Weizmann LeMada'') is a Public university, public research university in Rehovot, Israel, established in 1934, fourteen years before the State of Israel was founded. Unlike other List of Israeli uni ...
. It is on the World Health Organization's List of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health s ...
.
Medical uses
Glatiramer acetate isindicated
In medicine, an indication is a valid reason to use a certain test, medication, procedure, or surgery. There can be multiple indications to use a procedure or medication. An indication can commonly be confused with the term diagnosis. A diagnosis ...
for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
A 2010 Cochrane review concluded that glatiramer acetate had partial efficacy in "relapse-related clinical outcomes" but no effect on progression of the disease. As a result, it is approved by the US Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
(FDA) for reducing the frequency of relapses, but not for reducing the progression of disability.
Adverse effects
boxed warning
In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears near the beginning of the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administratio ...
about a rare but serious allergic reaction called anaphylaxis
Anaphylaxis (Greek: 'up' + 'guarding') is a serious, potentially fatal allergic reaction and medical emergency that is rapid in onset and requires immediate medical attention regardless of the use of emergency medication on site. It typicall ...
.
Side effects may include a lump at the injection site (injection site reaction
Injection site reactions (ISRs) are reactions that occur at the site of injection of a drug. They may be mild or severe and may or may not require medical intervention. Some reactions may appear immediately after injection, and some may be delayed ...
) in approximately 30% of users, and aches, fever, chills (flu-like symptoms) in approximately 10% of users. Side effect symptoms are generally mild in nature. A reaction that involves flushing, shortness of breath, anxiety and rapid heartbeat has been reported soon after injection in up to 5% of patients (usually after inadvertently injecting directly into a vein). These side effects subside within thirty minutes. Over time, a visible dent at a repeat-injection site can occur due to the local destruction of fat tissue, known as lipoatrophy
Lipoatrophy is the term describing the localized loss of fat tissue. This may occur as a result of subcutaneous injections of insulin in the treatment of diabetes, from the use of human growth hormone or from subcutaneous injections of copaxone u ...
, that may develop.
More serious side effects have been reported for glatiramer acetate, according to the prescription label in the US. These include serious side effects to the cardiovascular, digestive (including the liver), hematopoietic, lymphatic, musculoskeletal, nervous, respiratory, and urogenital systems as well as special senses (in particular the eyes). Metabolic and nutritional disorders have also been reported; however a link between glatiramer acetate and these adverse effects has not been established.
Mechanism of action
Glatiramer acetate is a random polymer (average molecular mass ) composed of fouramino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the Proteinogenic amino acid, 22 α-amino acids incorporated into p ...
found in myelin basic protein
Myelin basic protein (MBP) is a protein important in the process of myelination of nerves in the nervous system. The myelin sheath is a multi-layered membrane, unique to the nervous system, that functions as an insulator to greatly increase the ve ...
. The mechanism of action for glatiramer acetate is not fully elucidated. It is thought to act by modifying immune processes that are believed to be responsible for the pathogenesis of MS. Administration of glatiramer acetate shifts the population of T cells from proinflammatory Th1 T-cells to regulatory Th2 T-cells that suppress the inflammatory response. This is done by the inhibition of secretion of proinflammatory cytokines (IL-1, IL-12, TNF, INFγ) by Th1 T-cells, thereby inducing Th2 T-cells to cross the blood–brain barrier and produce anti-inflammatory cytokines (IL-4, IL-5, IL-13, IL-10, TGF-β). Given its resemblance to myelin basic protein, glatiramer acetate may act as a decoy, diverting an autoimmune response against myelin. This hypothesis is supported by findings of studies that have been carried out to explore the pathogenesis of experimental autoimmune encephalomyelitis
Experimental autoimmune encephalomyelitis, sometimes experimental allergic encephalomyelitis (EAE), is an animal model of brain inflammation. It is an inflammatory demyelinating disease of the central nervous system (CNS). It is mostly used with r ...
(EAE), a condition induced in several animal species through immunization against central nervous system derived material containing myelin and often used as an experimental animal model of MS. Studies in animals and in vitro systems suggest that upon its administration, glatiramer acetate-specific regulatory T cells
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain immune tolerance, tolerance to self-antigens, and prevent autoimmune disease. Treg ...
(Tregs) are induced and activated in the periphery, inhibiting the inflammatory reaction to myelin basic protein.
The integrity of the blood–brain barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system ...
, however, is not appreciably affected by glatiramer acetate, at least not in the early stages of treatment. Glatiramer acetate has been shown in clinical trials to reduce the number and severity of multiple sclerosis exacerbations.
History
Three main clinical trials demonstrated its safety and efficacy: The first trial was performed in a single center, double-blind, placebo controlled trial and included 50 patients. The second trial was a two-year, multi-center, randomized, double-blind, placebo controlled trial and involved 251 patients. The third trial was a double-blindMRI
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to generate pictures of the anatomy and the physiological processes inside the body. MRI scanners use strong magnetic fields, magnetic field gradients, and rad ...
study involving participation of 239 patients.
A 15-year followup of the original trial compared patients who continued with glatiramer to patients who dropped out of the trial. Patients with glatiramer had reduced relapse rates, and decreased disability progression and transition to secondary progressive MS, compared to patients who did not continue glatiramer. However, the two groups were not necessarily comparable, as it was no longer a randomized trial. There were no long-term safety issues.
Society and culture
Legal status
Glatiramer acetate has been approved in various countries, including the United States, Israel, Canada, and 24 European Union countries. Approval in the U.S. was obtained in 1997. Glatiramer acetate was approved in the UK in August 2000. This first approval in a major European market led to approval across the European Union under the mutual recognition procedure.Patent status
Novartis
Novartis AG is a Swiss multinational corporation, multinational pharmaceutical company, pharmaceutical corporation based in Basel, Switzerland. Novartis is one of the largest pharmaceutical companies in the world and was the eighth largest by re ...
subsidiary Sandoz has marketed Glatopa since 2015, a generic version of the original Teva 20 mg formulation that requires daily injection.
Teva developed a long-acting 40 mg formulation, marketed since 2015, which reduced required injections to three per week. In October 2017, the FDA approved a generic version, which is manufactured in India by Natco Pharma
Natco Pharma is an Indian multinational pharmaceutical company based in Hyderabad. The company manufactures finished dosage formulations, active pharmaceutical ingredients and agrochemical products. It is a major producer of branded oncology me ...
, and imported and sold by Dutch firm Mylan
Mylan N.V. was a global generic and specialty pharmaceuticals company. In November 2020, Mylan merged with Upjohn, Pfizer's off-patent medicine division, to form Viatris. Previously, the company was domiciled in the Netherlands, with principa ...
. In February 2018, Sandoz received FDA approval for their generic version. In parallel with the development and approval processes, the generic competitors have disputed Teva's newer patents, any of which if upheld, would prevent marketing of long-acting generics.
While the patent
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an sufficiency of disclosure, enabling discl ...
on the chemical drug expired in 2015, Teva obtained new US patents covering pharmaceutical formulations for long-acting delivery. Litigation from industry competitors in 2016-2017 resulted in the new patents being judged invalid. In October 2018, the U.S. Court of Appeals for the Federal Circuit
The United States Court of Appeals for the Federal Circuit (in case citations, Fed. Cir. or C.A.F.C.) is one of the 13 United States courts of appeals. It has special appellate jurisdiction over certain categories of cases in the U.S. federal ...
upheld the patent invalidation for obviousness
The inventive step and non-obviousness reflect a general patentability requirement present in most patent laws, according to which an invention should be sufficiently inventive—i.e., non-obvious—in order to be patented. In other words, " he ...
. The case reflects the larger controversy over evergreening
Evergreening is any of various legal, business, and technological strategies by which producers (often pharmaceutical companies) extend the lifetime of their patents that are about to expire in order to retain revenues from them. Often the practice ...
of branded drugs. On October 31, 2024, the European Commission
The European Commission (EC) is the primary Executive (government), executive arm of the European Union (EU). It operates as a cabinet government, with a number of European Commissioner, members of the Commission (directorial system, informall ...
fined Teva €462.6 million «over misuse of the patent system and disparagement to delay rival multiple sclerosis medicine», having attempted to obstacle other producers of glatiramer acetate both by abusing the patent system and by setting up a misinformation campaign targeting other glatiramer producers.
References
{{DEFAULTSORT:Glatiramer Acetate Immunostimulants Drugs with unknown mechanisms of action Israeli inventions World Health Organization essential medicines