Anodizing on:
[Wikipedia]
[Google]
[Amazon]
Anodizing is an electrolytic passivation process used to increase the thickness of the natural
 Aluminium alloys are anodized to increase corrosion resistance and to allow dyeing (coloring), improved lubrication, or improved
Aluminium alloys are anodized to increase corrosion resistance and to allow dyeing (coloring), improved lubrication, or improved
ASSIST database
more opaque films that are softer, ductile, and to a degree self-healing. They are harder to dye and may be applied as a pretreatment before painting. The method of film formation is different from using sulfuric acid in that the voltage is ramped up through the process cycle.
 Stainless steel can be anodized in baths containing sulphuric acid and hexavalent chromium compounds. Baths containing NaOH or KOH solutions can be used too.As hexavalent chromium compounds are prohibited for use in the EU based on ROHS regulations and are toxic and carcinogenic, solutions based on molybdate are proposed as a replacement (e.g. molybdate 30-100g/ boric acid 10-18 g/manganese sulfate 0.5 - 5 g/1 liter of water, 0.1 - 20 A/dm2, 0.1–15 minutes).
Stainless steel can be anodized in baths containing sulphuric acid and hexavalent chromium compounds. Baths containing NaOH or KOH solutions can be used too.As hexavalent chromium compounds are prohibited for use in the EU based on ROHS regulations and are toxic and carcinogenic, solutions based on molybdate are proposed as a replacement (e.g. molybdate 30-100g/ boric acid 10-18 g/manganese sulfate 0.5 - 5 g/1 liter of water, 0.1 - 20 A/dm2, 0.1–15 minutes).
 The most common anodizing processes, for example, sulphuric acid on aluminium, produce a porous surface which can accept dyes easily. The number of dye colors is almost endless; however, the colors produced tend to vary according to the base alloy. The most common colors in the industry, due to them being relatively cheap, are yellow, green, blue, black, orange, purple and red. Though some may prefer lighter colors, in practice they may be difficult to produce on certain alloys such as high-silicon casting grades and 2000-series aluminium-copper alloys. Another concern is the "lightfastness" of organic dyestuffs—some colors (reds and blues) are particularly prone to fading. Black dyes and gold produced by
The most common anodizing processes, for example, sulphuric acid on aluminium, produce a porous surface which can accept dyes easily. The number of dye colors is almost endless; however, the colors produced tend to vary according to the base alloy. The most common colors in the industry, due to them being relatively cheap, are yellow, green, blue, black, orange, purple and red. Though some may prefer lighter colors, in practice they may be difficult to produce on certain alloys such as high-silicon casting grades and 2000-series aluminium-copper alloys. Another concern is the "lightfastness" of organic dyestuffs—some colors (reds and blues) are particularly prone to fading. Black dyes and gold produced by 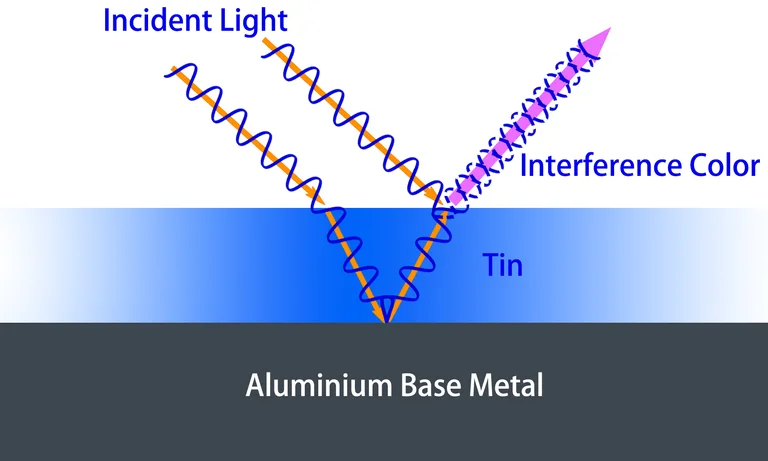 Another interesting coloring method is anodizing interference coloring. The thin oil film resting on the water's surface displays a rainbow hue due to the interference between light reflected from the water-oil interface and the oil film's surface. Because the oil film's thickness isn't regulated, the resulting rainbow color appears random.
In the anodizing coloring of aluminum, desired colors are achieved by depositing a controllably thick metal layer (typically tin) at the base of the porous structure. This involves reflections on the aluminum substrate and the upper metal surface. The color resulting from interference shifts from blue, green, and yellow to red as the deposited metal layer thickens. Beyond a specific thickness, the optical interference vanishes, and the color turns bronze. Interference-colored anodized aluminum parts exhibit a distinctive quality: their color varies when viewed from different angles. The interference coloring involves a 3-step process: sulfuric acid anodizing, electrochemical modification of the anodic pore, and metal (tin) deposition.
Another interesting coloring method is anodizing interference coloring. The thin oil film resting on the water's surface displays a rainbow hue due to the interference between light reflected from the water-oil interface and the oil film's surface. Because the oil film's thickness isn't regulated, the resulting rainbow color appears random.
In the anodizing coloring of aluminum, desired colors are achieved by depositing a controllably thick metal layer (typically tin) at the base of the porous structure. This involves reflections on the aluminum substrate and the upper metal surface. The color resulting from interference shifts from blue, green, and yellow to red as the deposited metal layer thickens. Beyond a specific thickness, the optical interference vanishes, and the color turns bronze. Interference-colored anodized aluminum parts exhibit a distinctive quality: their color varies when viewed from different angles. The interference coloring involves a 3-step process: sulfuric acid anodizing, electrochemical modification of the anodic pore, and metal (tin) deposition.
"Titanium in Technicolor"
an article on anodizing titanium from Theodore Gray's How2.0 column in ''
oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
layer on the surface of metal parts.
The process is called ''anodizing'' because the part to be treated forms the anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
electrode of an electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; ...
. Anodizing increases resistance to corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
and wear, and provides better adhesion for paint primers and glues than bare metal does. Anodic films can also be used for several cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add reflected light wave interference effects.
Anodizing is also used to prevent galling of threaded components and to make dielectric films for electrolytic capacitor
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
s. Anodic films are most commonly applied to protect aluminium alloys, although processes also exist for titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
, zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
, magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
, niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
, zirconium, hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
, and tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
. Iron or carbon steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
metal exfoliates when oxidized under neutral or alkaline micro-electrolytic conditions; i.e., the iron oxide
An iron oxide is a chemical compound composed of iron and oxygen. Several iron oxides are recognized. Often they are non-stoichiometric. Ferric oxyhydroxides are a related class of compounds, perhaps the best known of which is rust.
Iron ...
(actually ferric hydroxide or hydrated iron oxide, also known as rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO(OH) ...
) forms by anoxic anodic pits and large cathodic surface, these pits concentrate anions such as sulfate and chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
accelerating the underlying metal to corrosion. Carbon flakes or nodules in iron or steel with high carbon content ( high-carbon steel, cast iron
Cast iron is a class of iron–carbon alloys with a carbon content of more than 2% and silicon content around 1–3%. Its usefulness derives from its relatively low melting temperature. The alloying elements determine the form in which its car ...
) may cause an electrolytic potential and interfere with coating or plating. Ferrous metals are commonly anodized electrolytically in nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
or by treatment with red fuming nitric acid to form hard black Iron(II,III) oxide
Iron(II,III) oxide, or black iron oxide, is the chemical compound with formula Fe3O4. It occurs in nature as the mineral magnetite. It is one of a number of iron oxides, the others being iron(II) oxide (FeO), which is rare, and iron(III) oxide (Fe ...
. This oxide remains conformal even when plated on wiring and the wiring is bent.
Anodizing changes the microscopic texture of the surface and the crystal structure
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that repeat ...
of the metal near the surface. Thick coatings are normally porous, so a sealing process is often needed to achieve corrosion resistance. Anodized aluminium surfaces, for example, are harder than aluminium but have low to moderate wear resistance that can be improved with increasing thickness or by applying suitable sealing substances. Anodic films are generally much stronger and more adherent than most types of paint and metal plating, but also more brittle. This makes them less likely to crack and peel from ageing and wear, but more susceptible to cracking from thermal stress.
History
Anodizing was first used on an industrial scale in 1923 to protect Duraluminseaplane
A seaplane is a powered fixed-wing aircraft capable of takeoff, taking off and water landing, landing (alighting) on water.Gunston, "The Cambridge Aerospace Dictionary", 2009. Seaplanes are usually divided into two categories based on their tech ...
parts from corrosion. This early chromic acid
Chromic acid is a chemical compound with the chemical formula . It is also a jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.
The term "chromic ...
–based process was called the Bengough–Stuart process and was documented in British defence specification DEF STAN 03-24/3. It is still used today despite its legacy requirements for a complicated voltage cycle now known to be unnecessary. Variations of this process soon evolved, and the first sulfuric acid anodizing process was patented by Gower and O'Brien in 1927. Sulfuric acid soon became and remains the most common anodizing electrolyte.
Oxalic acid anodizing was first patented in Japan in 1923 and later widely used in Germany, particularly for architectural applications. Anodized aluminium extrusion was a popular architectural material in the 1960s and 1970s, but has since been displaced by cheaper plastic
Plastics are a wide range of synthetic polymers, synthetic or Semisynthesis, semisynthetic materials composed primarily of Polymer, polymers. Their defining characteristic, Plasticity (physics), plasticity, allows them to be Injection moulding ...
s and powder coating
Powder coating is a type of coating that is applied as a free-flowing, dry powder. Unlike conventional liquid paint, which is delivered via an evaporating solvent, powder coating is typically applied electrostatically and then Powder coating#Curin ...
.. The phosphoric acid processes are the most recent major development, so far only used as pretreatments for adhesives or organic paints.. A wide variety of proprietary and increasingly complex variations of all these anodizing processes continue to be developed by industry, so the growing trend in military and industrial standards is to classify by coating properties rather than by process chemistry.
Aluminium
 Aluminium alloys are anodized to increase corrosion resistance and to allow dyeing (coloring), improved lubrication, or improved
Aluminium alloys are anodized to increase corrosion resistance and to allow dyeing (coloring), improved lubrication, or improved adhesion
Adhesion is the tendency of dissimilar particles or interface (matter), surfaces to cling to one another. (Cohesion (chemistry), Cohesion refers to the tendency of similar or identical particles and surfaces to cling to one another.)
The ...
. However, anodizing does not increase the strength of the aluminium object. The anodic layer is insulative.
When exposed to air at room temperature, or any other gas containing oxygen, pure aluminium self-passivates by forming a surface layer of amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
aluminium oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several Aluminium oxide (compounds), aluminium oxides, and specifically identified as alum ...
2 to 3 nm thick, which provides very effective protection against corrosion. Aluminium alloys typically form a thicker oxide layer, 5–15 nm thick, but tend to be more susceptible to corrosion. Aluminium alloy parts are anodized to greatly increase the thickness of this layer for corrosion resistance. The corrosion resistance of aluminium alloys is significantly decreased by certain alloying elements or impurities: copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, and silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, so 2000-, 4000-, 6000 and 7000-series Al alloys tend to be most susceptible.
Although anodizing produces a very regular and uniform coating, microscopic fissures in the coating can lead to corrosion. Further, the coating is susceptible to chemical dissolution in the presence of high- and low- pH chemistry, which results in stripping the coating and corrosion of the substrate. To combat this, various techniques have been developed either to reduce the number of fissures, to insert more chemically stable compounds into the oxide, or both. For instance, sulphuric-anodized articles are normally sealed, either through hydro-thermal sealing or precipitating sealing, to reduce porosity and interstitial pathways that allow corrosive ion exchange between the surface and the substrate. Precipitating seals enhance chemical stability but are less effective in eliminating ionic exchange pathways. Most recently, new techniques to partially convert the amorphous oxide coating into more stable micro-crystalline compounds have been developed that have shown significant improvement based on shorter bond lengths.
Some aluminium aircraft parts, architectural materials, and consumer products are anodized. Anodized aluminium can be found on MP3 player
A portable media player (PMP) or digital audio player (DAP) is a portable consumer electronics device capable of storing and playing digital media such as audio, images, and video files. Normally they refer to small, battery-powered devices ...
s, smartphones
A smartphone is a mobile phone with advanced computing capabilities. It typically has a touchscreen interface, allowing users to access a wide range of applications and services, such as web browsing, email, and social media, as well as mult ...
, multi-tools, flashlights, cookware, camera
A camera is an instrument used to capture and store images and videos, either digitally via an electronic image sensor, or chemically via a light-sensitive material such as photographic film. As a pivotal technology in the fields of photograp ...
s, sporting goods, firearms
A firearm is any type of gun that uses an explosive charge and is designed to be readily carried and operated by an individual. The term is legally defined further in different countries (see legal definitions).
The first firearms originated ...
, window frames, roof
A roof (: roofs or rooves) is the top covering of a building, including all materials and constructions necessary to support it on the walls of the building or on uprights, providing protection against rain, snow, sunlight, extremes of tempera ...
s, in electrolytic capacitors, and on many other products both for corrosion resistance and the ability to retain dye. Although anodizing only has moderate wear resistance, the deeper pores can better retain a lubricating film than a smooth surface would.
Anodized coatings have a much lower thermal conductivity and coefficient of linear expansion than aluminium. As a result, the coating will crack from thermal stress
In mechanics and thermodynamics, thermal stress is mechanical stress created by any change in temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, m ...
if exposed to temperatures above 80 °C (353 K). The coating can crack, but it will not peel. The melting point of aluminium oxide is 2050 °C (2323K), much higher than pure aluminium's 658 °C (931K). This and the insulativity of aluminium oxide can make welding more difficult.
In typical commercial aluminium anodizing processes, the aluminium oxide is grown down into the surface and out from the surface by equal amounts. Therefore, anodizing will increase the part dimensions on each surface by half the oxide thickness. For example, a coating that is 2 μm thick will increase the part dimensions by 1 μm per surface. If the part is anodized on all sides, then all linear dimensions will increase by the oxide thickness. Anodized aluminium surfaces are harder than aluminium but have low to moderate wear resistance, although this can be improved with thickness and sealing.
Process
Desmut
A desmut solution can be applied to the surface of aluminium to remove contaminants. Nitric acid is typically used to remove smut (residue), but is being replaced because of environmental concerns.Electrolysis
The anodized aluminium layer is created by passing adirect current
Direct current (DC) is one-directional electric current, flow of electric charge. An electrochemical cell is a prime example of DC power. Direct current may flow through a conductor (material), conductor such as a wire, but can also flow throug ...
through an electrolytic solution, with the aluminium object serving as the anode (the positive electrode in an electrolytic cell). The current releases hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
at the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
(the negative electrode) and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
at the surface of the aluminium anode, creating a build-up of aluminium oxide. Alternating current
Alternating current (AC) is an electric current that periodically reverses direction and changes its magnitude continuously with time, in contrast to direct current (DC), which flows only in one direction. Alternating current is the form in w ...
and pulsed current is also possible but rarely used. The voltage required by various solutions may range from 1 to 300 V DC, although most fall in the range of 15 to 21 V. Higher voltages are typically required for thicker coatings formed in sulfuric and organic acid. The anodizing current varies with the area of aluminium being anodized and typically ranges from 30 to 300 A/ m2.
Aluminium anodizing (eloxal or Electrolytic Oxidation of Aluminium) is usually performed in an acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
ic solution, typically sulphuric acid or chromic acid, which slowly dissolves the aluminium oxide. The acid action is balanced with the oxidation rate to form a coating with nanopores, 10–150 nm in diameter. These pores are what allow the electrolyte solution and current to reach the aluminium substrate and continue growing the coating to greater thickness beyond what is produced by auto-passivation.. These pores allow for the dye to be absorbed, however, this must be followed by sealing or the dye will not stay. Dye is typically followed up by a clean nickel acetate seal. Because the dye is only superficial, the underlying oxide may continue to provide corrosion protection even if minor wear and scratches break through the dyed layer.
Conditions such as electrolyte concentration, acidity, solution temperature, and current must be controlled to allow the formation of a consistent oxide layer. Harder, thicker films tend to be produced by more concentrated solutions at lower temperatures with higher voltages and currents. The film thickness can range from under 0.5 micrometers for bright decorative work up to 150 micrometers for architectural applications.
Dual-finishing
Anodizing can be performed in combination withchromate conversion coating
Chromate conversion coating or alodine coating is a type of conversion coating used to passivate steel, aluminium, zinc, cadmium, copper, silver, titanium, magnesium, and tin alloys. The coating serves as a corrosion inhibitor, as a pri ...
. Each process provides corrosion resistance, with anodizing offering a significant advantage when it comes to ruggedness or physical wear resistance. The reason for combining the processes can vary, however, the significant difference between anodizing and chromate conversion coating is the electrical conductivity of the films produced. Although both stable compounds, chromate conversion coating has a greatly increased electrical conductivity. Applications where this may be useful are varied, however the issue of grounding components as part of a larger system is an obvious one.
The dual finishing process uses the best each process has to offer, anodizing with its hard wear resistance and chromate conversion coating with its electrical conductivity.
The process steps can typically involve chromate conversion coating the entire component, followed by a masking of the surface in areas where the chromate coating must remain intact. Beyond that, the chromate coating is then dissolved in unmasked areas. The component can then be anodized, with anodizing taking to the unmasked areas. The exact process will vary dependent on service provider, component geometry and required outcome. It helps to protect aluminium article.
Widely used specifications
The most widely used anodizing specification in the US is a U.S. military spec, MIL-A-8625, which defines three types of aluminium anodizing. Type I is chromic acid anodizing, Type II is sulphuric acid anodizing, and Type III is sulphuric acid hard anodizing. Other anodizing specifications include more MIL-SPECs (e.g., MIL-A-63576), aerospace industry specs by organizations such as SAE,ASTM
ASTM International, formerly known as American Society for Testing and Materials, is a standards organization that develops and publishes voluntary consensus technical international standards for a wide range of materials, products, systems and s ...
, and ISO
The International Organization for Standardization (ISO ; ; ) is an independent, non-governmental, international standard development organization composed of representatives from the national standards organizations of member countries.
Me ...
(e.g., AMS 2469, AMS 2470, AMS 2471, AMS 2472, AMS 2482, ASTM B580, ASTM D3933, ISO 10074, and BS 5599), and corporation-specific specs (such as those of Boeing, Lockheed Martin, Airbus and other large contractors). AMS 2468 is obsolete. None of these specifications define a detailed process or chemistry, but rather a set of tests and quality assurance measures which the anodized product must meet. BS 1615 guides the selection of alloys for anodizing. For British defense work, a detailed chromic and sulfuric anodizing processes are described by DEF STAN 03-24/3 and DEF STAN 03-25/3 respectively.
Chromic acid (Type I)
The oldest anodizing process useschromic acid
Chromic acid is a chemical compound with the chemical formula . It is also a jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.
The term "chromic ...
. It is widely known as the Bengough-Stuart process but, due to the safety regulations regarding air quality control, is not preferred by vendors when the additive material associated with type II doesn't break tolerances. In North America, it is known as Type I because it is so designated by the MIL-A-8625 standard, but it is also covered by AMS 2470 and MIL-A-8625 Type IB. In the UK it is normally specified as Def Stan 03/24 and used in areas that are prone to come into contact with propellants etc. There are also Boeing and Airbus standards. Chromic acid produces thinner, 0.5 μm to 18 μm (0.00002" to 0.0007")US Military Specification MIL-A-8625ASSIST database
more opaque films that are softer, ductile, and to a degree self-healing. They are harder to dye and may be applied as a pretreatment before painting. The method of film formation is different from using sulfuric acid in that the voltage is ramped up through the process cycle.
Sulfuric acid (Type II & III)
Sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
is the most widely used solution to produce an anodized coating. Coatings of moderate thickness 1.8 μm to 25 μm (0.00007" to 0.001") are known as Type II in North America, as named by MIL-A-8625, while coatings thicker than 25 μm (0.001") are known as Type III, hard-coat, hard anodizing, or engineered anodizing. Very thin coatings similar to those produced by chromic anodizing are known as Type IIB. Thick coatings require more process control, and are produced in a refrigerated tank near the freezing point of water with higher voltages than the thinner coatings. Hard anodizing can be made between 13 and 150 μm (0.0005" to 0.006") thick. Anodizing thickness increases wear resistance, corrosion resistance, ability to retain lubricants and PTFE
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off fro ...
coatings, and electrical and thermal insulation. Sealing Type III will improve corrosion resistance at the cost of reducing abrasion resistance. Sealing will reduce this greatly. Standards for thin (Soft/Standard) sulfuric anodizing are given by MIL-A-8625 Types II and IIB, AMS 2471 (undyed), and AMS 2472 (dyed), BS EN ISO 12373/1 (decorative), BS 3987 (Architectural). Standards for thick sulphuric anodizing are given by MIL-A-8625 Type III, AMS 2469, BS ISO 10074, BS EN 2536 and the obsolete AMS 2468 and DEF STAN 03-26/1.
Organic acid
Anodizing can produce yellowish integral colors without dyes if it is carried out in weak acids with high voltages, high current densities, and strong refrigeration. Shades of color are restricted to a range which includes pale yellow, gold, deep bronze, brown, grey, and black. Some advanced variations can produce a white coating with 80% reflectivity. The shade of color produced is sensitive to variations in the metallurgy of the underlying alloy and cannot be reproduced consistently. Anodizing in some organic acids, for examplemalic acid
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms ( ...
, can enter a 'runaway' situation, in which the current drives the acid to attack the aluminium far more aggressively than normal, resulting in huge pits and scarring. Also, if the current or voltage are driven too high, 'burning' can set in; in this case, the supplies act as if nearly shorted and large, uneven and amorphous black regions develop.
Integral color anodizing is generally done with organic acids, but the same effect has been produced in laboratories with very dilute sulfuric acid. Integral color anodizing was originally performed with oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name i ...
, but sulfonated aromatic compounds containing oxygen, particularly sulfosalicylic acid, have been more common since the 1960s. Thicknesses of up to 50 μm can be achieved. Organic acid anodizing is called Type IC by MIL-A-8625.
Phosphoric acid
Anodizing can be carried out in phosphoric acid, usually as a surface preparation for adhesives. This is described in standard ASTM D3933.Borate and tartrate baths
Anodizing can also be performed inborate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of su ...
or tartrate baths in which aluminium oxide is insoluble. In these processes, the coating growth stops when the part is fully covered, and the thickness is linearly related to the voltage applied. These coatings are free of pores, relative to the sulfuric and chromic acid processes. This type of coating is widely used to make electrolytic capacitors because the thin aluminium films (typically less than 0.5 μm) would risk being pierced by acidic processes.
Plasma electrolytic oxidation
Plasma electrolytic oxidation is a similar process, but where higher voltages are applied. This causes sparks to occur and results in more crystalline/ceramic type coatings.Other metals
Magnesium
Magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
is anodized primarily as a primer for paint. A thin (5 μm) film is sufficient for this. Thicker coatings of 25 μm and up can provide mild corrosion resistance when sealed with oil, wax, or sodium silicate. Standards for magnesium anodizing are given in AMS 2466, AMS 2478, AMS 2479, and ASTM B893.
Niobium
Niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
anodizes in a similar fashion to titanium with a range of attractive colors being formed by interference at different film thicknesses. Again the film thickness is dependent on the anodizing voltage. Uses include jewelry
Jewellery (or jewelry in American English) consists of decorative items worn for personal adornment such as brooches, ring (jewellery), rings, necklaces, earrings, pendants, bracelets, and cufflinks. Jewellery may be attached to the body or the ...
and commemorative coin
A commemorative coin is a coin issued to commemorate some particular event or issue with a distinct design with reference to the occasion on which they were issued. Some coins of this category serve as collector's items only, while most commemora ...
s.
Stainless steel
 Stainless steel can be anodized in baths containing sulphuric acid and hexavalent chromium compounds. Baths containing NaOH or KOH solutions can be used too.As hexavalent chromium compounds are prohibited for use in the EU based on ROHS regulations and are toxic and carcinogenic, solutions based on molybdate are proposed as a replacement (e.g. molybdate 30-100g/ boric acid 10-18 g/manganese sulfate 0.5 - 5 g/1 liter of water, 0.1 - 20 A/dm2, 0.1–15 minutes).
Stainless steel can be anodized in baths containing sulphuric acid and hexavalent chromium compounds. Baths containing NaOH or KOH solutions can be used too.As hexavalent chromium compounds are prohibited for use in the EU based on ROHS regulations and are toxic and carcinogenic, solutions based on molybdate are proposed as a replacement (e.g. molybdate 30-100g/ boric acid 10-18 g/manganese sulfate 0.5 - 5 g/1 liter of water, 0.1 - 20 A/dm2, 0.1–15 minutes).
Tantalum
Tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
anodizes similarly to titanium and niobium with a range of attractive colors being formed by interference at different film thicknesses. Again the film thickness is dependent on the anodizing voltage and typically ranges from 18 to 23 Angstroms per volt depending on electrolyte and temperature. Uses include tantalum capacitors.
Titanium
An anodized oxide layer has a thickness in the range of to several micrometers. Standards for titanium anodizing are given by AMS 2487 and AMS 2488. AMS 2488 Type III anodizing of titanium generates an array of different colors without dyes, for which it is sometimes used in art, costume jewellery, body piercing jewellery and wedding rings. The color formed is dependent on the thickness of the oxide (which is determined by the anodizing voltage); it is caused by the interference of light reflecting off the oxide surface with light travelling through it and reflecting off the underlying metal surface. AMS 2488 Type II anodizing produces a thicker matte grey finish with higher wear resistance.Zinc
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
is rarely anodized, but a process was developed by the International Lead Zinc Research Organization and covered by MIL-A-81801. A solution of ammonium phosphate, chromate and fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
with voltages of up to 200 V can produce olive green coatings up to 80 μm thick. The coatings are hard and corrosion resistant.
Zinc or galvanized steel can be anodized using DC at lower voltages (20–30 V) in silicate baths containing varying concentrations of sodium silicate, sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
, borax, sodium nitrite, and nickel sulfate.
Dyeing
 The most common anodizing processes, for example, sulphuric acid on aluminium, produce a porous surface which can accept dyes easily. The number of dye colors is almost endless; however, the colors produced tend to vary according to the base alloy. The most common colors in the industry, due to them being relatively cheap, are yellow, green, blue, black, orange, purple and red. Though some may prefer lighter colors, in practice they may be difficult to produce on certain alloys such as high-silicon casting grades and 2000-series aluminium-copper alloys. Another concern is the "lightfastness" of organic dyestuffs—some colors (reds and blues) are particularly prone to fading. Black dyes and gold produced by
The most common anodizing processes, for example, sulphuric acid on aluminium, produce a porous surface which can accept dyes easily. The number of dye colors is almost endless; however, the colors produced tend to vary according to the base alloy. The most common colors in the industry, due to them being relatively cheap, are yellow, green, blue, black, orange, purple and red. Though some may prefer lighter colors, in practice they may be difficult to produce on certain alloys such as high-silicon casting grades and 2000-series aluminium-copper alloys. Another concern is the "lightfastness" of organic dyestuffs—some colors (reds and blues) are particularly prone to fading. Black dyes and gold produced by inorganic
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''.
Inor ...
means ( ferric ammonium oxalate) are more lightfast. Dyed anodizing is usually sealed to reduce or eliminate dye bleed out. White color cannot be applied due to the larger molecule size than the pore size of the oxide layer.
Alternatively, metal (usually tin) can be electrolytically deposited in the pores of the anodic coating to provide more lightfast colors. Metal dye colors range from pale champagne
Champagne (; ) is a sparkling wine originated and produced in the Champagne wine region of France under the rules of the appellation, which demand specific vineyard practices, sourcing of grapes exclusively from designated places within it, spe ...
to black
Black is a color that results from the absence or complete absorption of visible light. It is an achromatic color, without chroma, like white and grey. It is often used symbolically or figuratively to represent darkness.Eva Heller, ''P ...
. Bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals (such as phosphorus) or metalloid ...
shades are commonly used for architectural metals. Alternatively, the color may be produced integral to the film. This is done during the anodizing process using organic acids mixed with the sulfuric electrolyte and a pulsed current.
Splash effects are created by dying the unsealed porous surface in lighter colors and then splashing darker color dyes onto the surface. Aqueous and solvent-based dye mixtures may also be alternately applied since the colored dyes will resist each other and leave spotted effects.
 Another interesting coloring method is anodizing interference coloring. The thin oil film resting on the water's surface displays a rainbow hue due to the interference between light reflected from the water-oil interface and the oil film's surface. Because the oil film's thickness isn't regulated, the resulting rainbow color appears random.
In the anodizing coloring of aluminum, desired colors are achieved by depositing a controllably thick metal layer (typically tin) at the base of the porous structure. This involves reflections on the aluminum substrate and the upper metal surface. The color resulting from interference shifts from blue, green, and yellow to red as the deposited metal layer thickens. Beyond a specific thickness, the optical interference vanishes, and the color turns bronze. Interference-colored anodized aluminum parts exhibit a distinctive quality: their color varies when viewed from different angles. The interference coloring involves a 3-step process: sulfuric acid anodizing, electrochemical modification of the anodic pore, and metal (tin) deposition.
Another interesting coloring method is anodizing interference coloring. The thin oil film resting on the water's surface displays a rainbow hue due to the interference between light reflected from the water-oil interface and the oil film's surface. Because the oil film's thickness isn't regulated, the resulting rainbow color appears random.
In the anodizing coloring of aluminum, desired colors are achieved by depositing a controllably thick metal layer (typically tin) at the base of the porous structure. This involves reflections on the aluminum substrate and the upper metal surface. The color resulting from interference shifts from blue, green, and yellow to red as the deposited metal layer thickens. Beyond a specific thickness, the optical interference vanishes, and the color turns bronze. Interference-colored anodized aluminum parts exhibit a distinctive quality: their color varies when viewed from different angles. The interference coloring involves a 3-step process: sulfuric acid anodizing, electrochemical modification of the anodic pore, and metal (tin) deposition.
Sealing
Sealing is the final step in the anodizing process. Acidic anodizing solutions produce pores in the anodized coating. These pores can absorb dyes and retain lubricants but are also an avenue for corrosion. When lubrication properties are not critical, they are usually sealed after dyeing to increase corrosion resistance and dye retention. There are three most common types of sealing. # Long immersion in boiling-hot——deionized water or steam is the simplest sealing process, although it is not completely effective and reduces abrasion resistance by 20%. The oxide is converted into its hydrated form and the resulting swelling reduces the porosity of the surface. # Mid-temperature sealing process which works at in solutions containing organic additives and metal salts. However, this process will likely leach the colors. # Cold sealing process, where the pores are closed by impregnation of a sealant in a room-temperature bath, is more popular due to energy savings. Coatings sealed in this method are not suitable for adhesive bonding. Teflon, nickel acetate, cobalt acetate, and hot sodium or potassium dichromate seals are commonly used. MIL-A-8625 requires sealing for thin coatings (Types I and II) and allows it as an option for thick ones (Type III).Cleaning
Anodized aluminium surfaces that are not regularly cleaned are susceptible to panel edge staining, a unique type of surface staining that can affect the structural integrity of the metal.Environmental impact
Anodizing is one of the more environmentally friendly metal finishing processes. Except for organic (aka integral color) anodizing, the by-products contain only small amounts ofheavy metals
upright=1.2, Crystals of lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals is a controversial and ambiguous term for metallic elements with relatively h ...
, halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s, or volatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapor pressure at room temperature. They are common and exist in a variety of settings and products, not limited to Indoor mold, house mold, Upholstery, upholstered furnitur ...
s. Integral color anodizing produces no VOCs, heavy metals, or halogens as all of the byproducts found in the effluent streams of other processes come from their dyes or plating materials. The most common anodizing effluents, aluminium hydroxide and aluminium sulfate
Aluminium sulfate is a salt with the chemical formula, formula . It is soluble in water and is mainly used as a Coagulation (water treatment), coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking ...
, are recycled for the manufacturing of alum, baking powder, cosmetics, newsprint and fertilizer or used by industrial wastewater treatment
Industrial wastewater treatment describes the processes used for Wastewater treatment, treating wastewater that is produced by industries as an undesirable by-product. After treatment, the treated industrial wastewater (or effluent) may be reus ...
systems.
Mechanical considerations
Anodizing will raise the surface since the oxide created occupies more space than the base metal converted. This will generally not be of consequence except where there are tight tolerances. If so, the thickness of the anodizing layer has to be taken into account when choosing the machining dimension. A general practice on engineering drawing is to specify that "dimensions apply after all surface finishes". This will force the machine shop to take into account the anodization thickness when performing the final machining of the mechanical part before anodization. Also in the case of small holes threaded to acceptscrew
A screw is an externally helical threaded fastener capable of being tightened or released by a twisting force (torque) to the screw head, head. The most common uses of screws are to hold objects together and there are many forms for a variety ...
s, anodizing may cause the screws to bind, thus the threaded holes may need to be chased with a tap to restore the original dimensions. Alternatively, special oversize taps may be used to precompensate for this growth. In the case of unthreaded holes that accept fixed-diameter pins or rods, a slightly oversized hole to allow for the dimension change may be appropriate. Depending on the alloy and thickness of the anodized coating, the same may have a significantly negative effect on fatigue life. Conversely, anodizing may increase fatigue life by preventing corrosion pitting.
See also
* Black oxide * Phosphate conversion coating * Electrochemical coloring of metalsReferences
Citations
Bibliography
* *External links
"Titanium in Technicolor"
an article on anodizing titanium from Theodore Gray's How2.0 column in ''
Popular Science
Popular science (also called pop-science or popsci) is an interpretation of science intended for a general audience. While science journalism focuses on recent scientific developments, popular science is more broad ranging. It may be written ...
''
{{Authority control
Coatings
Corrosion prevention
Electrolysis
Metallurgical processes