allyl on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic
A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic
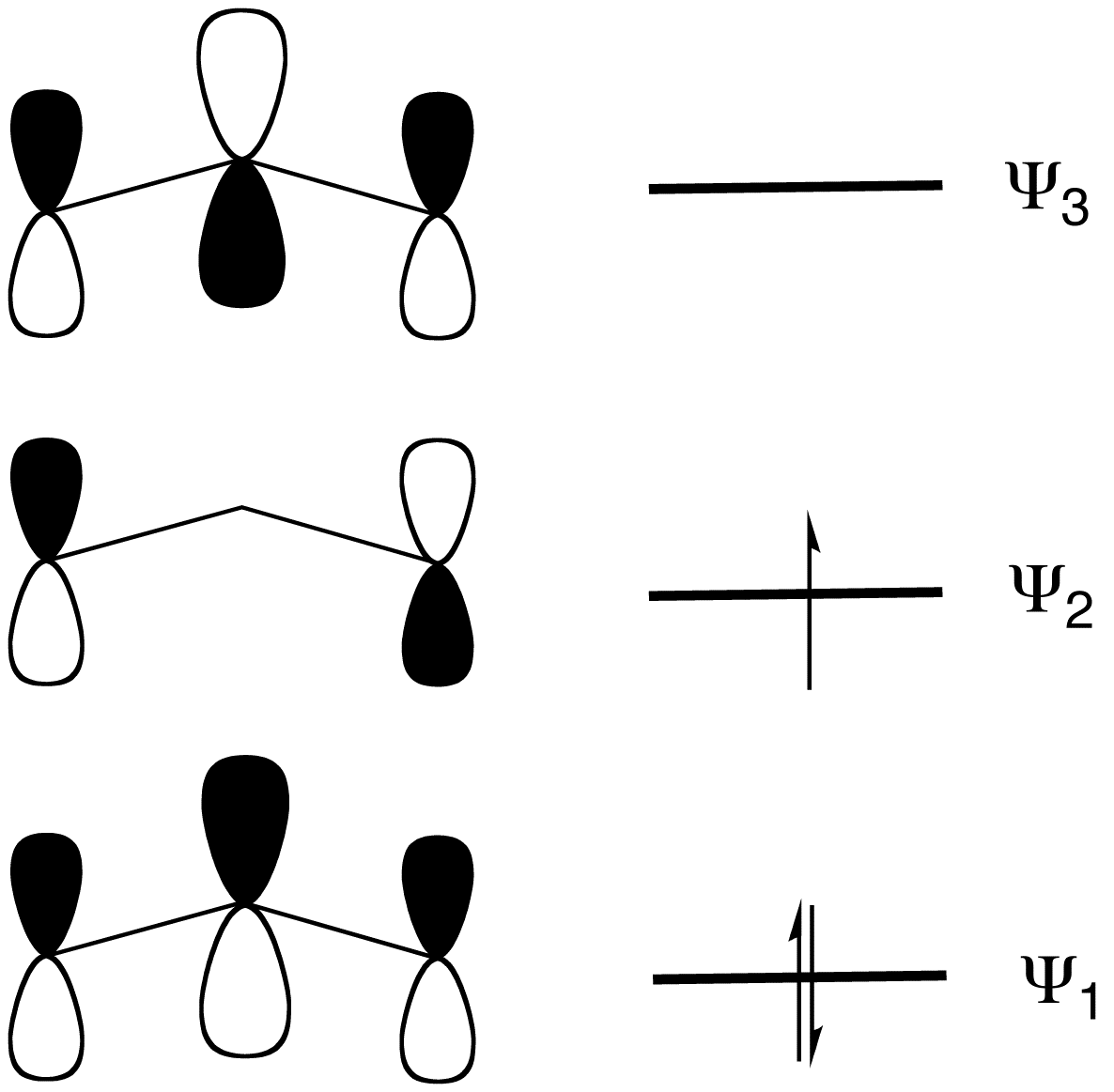
 In terms of MO theory, the MO diagram has three molecular orbitals: the first one bonding, the second one non-bonding, and the higher energy orbital is antibonding.
:
In terms of MO theory, the MO diagram has three molecular orbitals: the first one bonding, the second one non-bonding, and the higher energy orbital is antibonding.
:
2CH3-CH=CH2 + 2 NH3 + 3 O2 -> 2CH2=CH-C#N + 6 H2O
An estimated 800,000 tonnes (1997) of CH3CH=CH2 + Cl2 -> ClCH2CH=CH2 + HCl
It is the precursor to allyl alcohol and
 In the synthesis of some fine chemicals,
In the synthesis of some fine chemicals,
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, an allyl group is a substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
with the structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are connected to one another. The chemical bonding within the molecule is al ...
. It consists of a methylene bridge
In chemistry, a methylene bridge is part of a molecule with formula . The carbon atom is connected by single bonds to two other distinct atoms in the rest of the molecule. A methylene bridge is often called a methylene group or simply methylene, ...
() attached to a vinyl group (). The name is derived from the scientific name for garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plants in the genus '' Allium''. Its close relatives include the onion, shallot, leek, chives, Welsh onion, and Chinese onion. Garlic is native to central and south Asia, str ...
, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride
Allyl chloride is the organic compound with the formula C H2=CHCH2 Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a c ...
.
Allylation is any chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
that adds an allyl group to a substrate
Substrate may refer to:
Physical layers
*Substrate (biology), the natural environment in which an organism lives, or the surface or medium on which an organism grows or is attached
** Substrate (aquatic environment), the earthy material that exi ...
.
Nomenclature
hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive.
Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with allylic compounds are allylic oxidation
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isola ...
s, ene reactions, and the Tsuji–Trost reaction. Benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group ().
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substituent ...
ic groups are related to allyl groups; both show enhanced reactivity.
Pentadienyl group
A group connected to two vinyl groups is said to be doubly allylic. Thebond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical ...
of C−H bonds on a doubly allylic centre is about 10% less than the bond dissociation energy of a C−H bond that is singly allylic. The weakened C−H bonds is reflected in the easy oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of compounds containing 1,4-pentadiene
In organic chemistry, pentadiene is any hydrocarbon with an open Catenation, chain of five carbons, connected by two single bonds and two double bonds. All those compounds have the same molecular formula . The inventory of pentadienes include:
* 1 ...
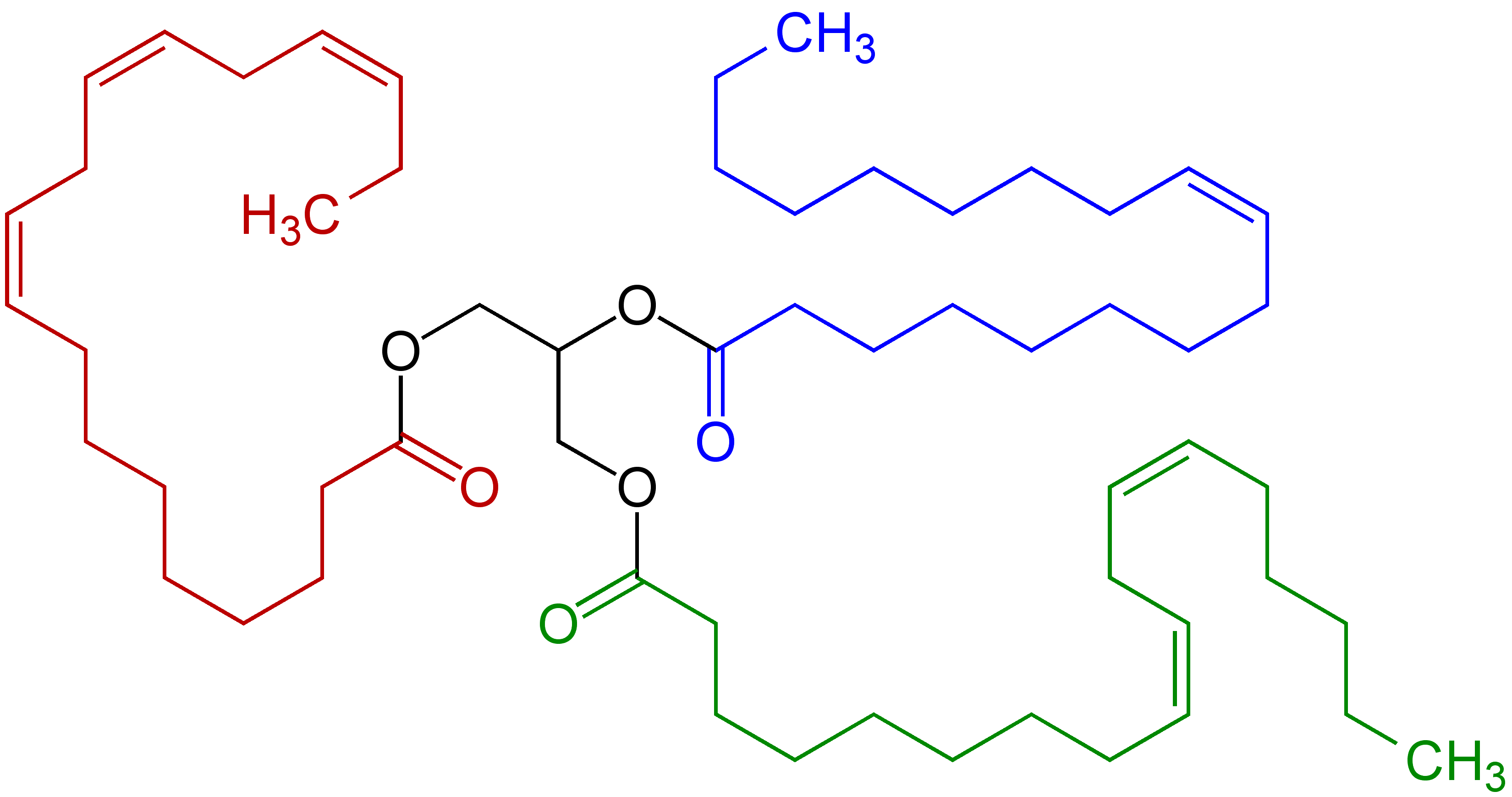
() linkages. Some polyunsaturated fatty acid
In biochemistry and nutrition, a polyunsaturated fat is a fat that contains a polyunsaturated fatty acid (abbreviated PUFA), which is a subclass of fatty acid characterized by a backbone with two or more carbon–carbon double bonds.
Some polyunsa ...
s feature this pentadiene group: linoleic acid
Linoleic acid (LA) is an organic compound with the formula . Both alkene groups () are ''cis''. It is a fatty acid sometimes denoted 18:2 (n−6) or 18:2 ''cis''-9,12. A linoleate is a salt or ester of this acid.
Linoleic acid is a polyunsat ...
, α-linolenic acid
Linolenic acid is a type of naturally-occurring fatty acid. It can refer to either of two octadecatrienoic acids (i.e. with an 18-carbon chain and three double bonds, which are found in the '' cis'' configuration), or a mixture of the two. Lino ...
, and arachidonic acid
Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega−6 fatty acid 20:4(ω−6), or 20:4(5,8,11,14). It is a precursor in the formation of leukotrienes, prostaglandins, and thromboxanes.
Together with omega−3 fatty acids an ...
. They are susceptible to a range of reactions with oxygen (O2), starting with lipid peroxidation
Lipid peroxidation, or lipid oxidation, is a complex chemical process that leads to oxidative degradation of lipids, resulting in the formation of peroxide and hydroperoxide derivatives.{{Cite journal , last1=Ayala , first1=Antonio , last2=Muñoz ...
. Products include fatty acid hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds of the form ROOH, where R stands for any group, typically Organic compound, organic, which contain the hydroperoxy functional group (). Hydroperoxide also refers to the hydroperoxide anio ...
s, epoxy-hydroxy polyunsaturated fatty acids, jasmonate
Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herb ...
s, divinylether fatty acids, and leaf aldehydes. Some of these derivatives are signallng molecules, some are used in plant defense (antifeedant
Antifeedants are organic compounds produced by plants to repel herbivores through distaste or toxicity. These chemical compounds are typically classified as secondary metabolites in that they are not essential for the metabolism of the plant, but ...
s), some are precursors to other metabolites that are used by the plant.
One practical consequence of their high reactivity is that polyunsaturated fatty acids have poor shelf life owing to their tendency toward autoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organi ...
, leading, in the case of edibles, to rancidification
Rancidification is the process of complete or incomplete autoxidation or hydrolysis of fats and oils when exposed to air, light, moisture, or bacterial action, producing short-chain aldehydes, ketones and free fatty acids.
When these processes ...
. Metals accelerate the degradation. These fats tend to polymerize, forming semisolids. This reactivity pattern is fundamental to the film-forming behavior of the "drying oil
Drying is a mass transfer process consisting of the removal of water or another solvent by evaporation from a solid, semi-solid or liquid. This process is often used as a final production step before selling or packaging products. To be conside ...
s", which are components of oil paint
Oil paint is a type of slow-drying paint that consists of particles of pigment suspended in a drying oil, commonly linseed oil. Oil paint also has practical advantages over other paints, mainly because it is waterproof.
The earliest surviving ...
s and varnish
Varnish is a clear Transparency (optics), transparent hard protective coating or film. It is not to be confused with wood stain. It usually has a yellowish shade due to the manufacturing process and materials used, but it may also be pigmente ...
es.

Homoallylic
The term homoallylic refers to the position on a carbon skeleton next to an allylic position. In but-3-enyl chloride , the chloride is homoallylic because it is bonded to the homoallylic site.Bonding
The allyl group is widely encountered in organic chemistry.Jerry March, "Advanced Organic Chemistry" 4th Ed. J. Wiley and Sons, 1992: New York. . Allylicradicals
Radical (from Latin: ', root) may refer to:
Politics and ideology Politics
*Classical radicalism, the Radical Movement that began in late 18th century Britain and spread to continental Europe and Latin America in the 19th century
*Radical politics ...
, anions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
, and cations
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
are often discussed as intermediates in reactions. All feature three contiguous sp²-hybridized carbon centers and all derive stability from resonance.Organic Chemistry John McMurry 2nd ed. 1988 Each species can be presented by two resonance structures with the charge or unpaired electron distributed at both 1,3 positions.
:
Reactions and applications
This heightened reactivity of allylic groups has many practical consequences. Thesulfur vulcanization
Sulfur vulcanization is a chemical process for converting natural rubber or related polymers into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cros ...
or various rubbers exploits the conversion of allylic groups into crosslinks. Similarly drying oil
Drying is a mass transfer process consisting of the removal of water or another solvent by evaporation from a solid, semi-solid or liquid. This process is often used as a final production step before selling or packaging products. To be conside ...
s such as linseed oil
Linseed oil, also known as flaxseed oil or flax oil (in its edible form), is a colorless to yellowish oil obtained from the dried, ripened seeds of the flax plant (''Linum usitatissimum''). The oil is obtained by pressing, sometimes followed by ...
crosslink via oxygenation of allylic (or doubly allylic) sites. This crosslinking underpins the properties of paints and the spoilage of foods by rancidification
Rancidification is the process of complete or incomplete autoxidation or hydrolysis of fats and oils when exposed to air, light, moisture, or bacterial action, producing short-chain aldehydes, ketones and free fatty acids.
When these processes ...
.
The industrial production of acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid. It has a pungent odor of garlic or onions. Its molecular structure consists of a vinyl group () linked to a nitrile (). It is an im ...
by ammoxidation
In organic chemistry, ammoxidation is a process for the production of nitriles () using ammonia () and oxygen (). It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrate ...
of propene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like od ...
exploits the easy oxidation of the allylic C−H centers:
:allyl chloride
Allyl chloride is the organic compound with the formula C H2=CHCH2 Cl. This colorless liquid is insoluble in water but soluble in common organic solvents. It is mainly converted to epichlorohydrin, used in the production of plastics. It is a c ...
is produced by the chlorination of propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like o ...
:
:epichlorohydrin
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscibility, miscible with most p ...
.
Allylation
Allylation is the attachment of an allyl group to a substrate, usually another organic compound. Classically, allylation involves the reaction of acarbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
with allyl chloride. Alternatives include carbonyl allylation
In organic chemistry, carbonyl allylation describes methods for adding an allyl anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaytsev (chemist), Alexander Zaitsev an ...
with allylmetallic reagents, such as allyltrimethylsilane, or the iridium-catalyzed Krische allylation.
Allylation can be effected also by conjugate addition: the addition of an allyl group to the beta-position of an enone. The Hosomi-Sakurai reaction is a common method for conjugate allylation.

Oxidation
Allylic C-H bonds are susceptible to oxidation. One commercial application of allylic oxidation is the synthesis ofnootkatone
Nootkatone is an organic compound, a sesquiterpenoid, which means that it is a C15 derivative that also contains an oxygen-containing functional group (a ketone). It is the most valuable aroma compound of grapefruit. Nootkatone was originally isol ...
, the fragrance of grapefruit
The grapefruit (''Citrus'' × ''paradisi'') is a subtropical citrus tree known for its relatively large, sour to semi-sweet, somewhat bitter fruit. The flesh of the fruit is segmented and varies in color from pale yellow to dark red.
Grapefru ...
, from valencene, a more abundantly available sesquiterpenoid
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many combinations. Biochemical modifications such ...
:
selenium dioxide
Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium. It is used in making specialized glasses as well as a reagent in organic chemistry.
Properties ...
is used to convert alkenes to allylic alcohols:
:R2C=CR'-CHR"2 + → R2C=CR'-C(OH)R"2
where R, R', R" may be alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
substituents.
From the industrial perspective, oxidation of benzylic C-H bonds are conducted on a particularly large scale, e.g. production of terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tons are produced annuall ...
, benzoic acid
Benzoic acid () is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. The benzoyl group is often abbreviated "Bz" (not to be confused with "Bn," which ...
, and cumene hydroperoxide
Cumene hydroperoxide is the organic compound with the formula C6H5C(CH3)2OOH; this oily liquid is classified as an organic hydroperoxide. Products of decomposition of cumene hydroperoxide are methylstyrene, acetophenone, and 2-phenylpropan-2- ...
.
Allyl compounds
Many substituents can be attached to the allyl group to give stable compounds. Commercially important allyl compounds include: * Crotyl alcohol (CH3CH=CH−CH2OH) *Dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynt ...
, central in the biosynthesis of terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...
s, a precursor to many natural products, including natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
.
* Transition-metal allyl complexes, such as allylpalladium chloride dimer
See also
* Propargylic/Homopropargylic * Benzylic *Vinylic
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound contai ...
* Acetylenic In organic chemistry, the term acetylenic designates
*A doubly unsaturated position (''sp''-hybridized) on a molecular framework, for instance in an alkyne such as acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemi ...
* Allylic strain
250 px, Allylic strain in an olefin.
Allylic strain (also known as A1,3 strain, 1,3-allylic strain, or A-strain) in organic chemistry is a type of strain energy resulting from the interaction between a substituent on one end of an olefin (a synony ...
* Allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction, organic chemical reaction in which reaction at a center Vicinal (chemistry), vicinal to a double bond causes the double bond to shift to an adjacent pair of atoms:
It is encountered ...
* Carroll rearrangement
* Allylic palladium complex
* Tsuji–Trost reaction
* Naloxone
Naloxone, sold under the brand name Narcan among others, is an opioid antagonist, a medication used to reverse or reduce the effects of opioids. For example, it is used to restore breathing after an opioid overdose. Effects begin within two ...
References
{{Authority control Alkenyl groups Allyl compounds