|
Benzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group (). Nomenclature In IUPAC nomenclature, the prefix benzyl refers to a substituent, for example benzyl chloride or benzyl benzoate. Benzyl is not to be confused with phenyl with the formula . The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring. For example, is referred to as a "benzylic" carbocation. The benzyl free radical has the formula . The benzyl cation or phenylcarbenium ion is the carbocation with formula ; the benzyl anion or phenylmethanide ion is the carbanion with the formula . None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanisms and may exist as reactive intermediates. Abbreviations Benzyl is most commonly abbreviated Bn. For ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wohl–Ziegler Bromination
The Wohl–Ziegler reaction is a chemical reaction that involves the allylic or benzylic bromination of hydrocarbons using an ''N''-bromosuccinimide and a radical initiator. : Best yields are achieved with ''N''-bromosuccinimide in carbon tetrachloride solvent. Several reviews have been published. In a typical setup, a stoichiometric amount of ''N''-bromosuccinimide solution and a small quantity of initiator are added to a solution of the substrate in CCl4, and the reaction mixture is stirred and heated to the boiling point. Initiation of the reaction is indicated by more vigorous boiling; sometimes the heat source may need to be removed. Once all ''N''-bromosuccinimide (which is denser than the solvent) has been converted to succinimide (which floats on top) the reaction has finished. Due to the high toxicity and ozone-depleting nature of carbon tetrachloride, trifluorotoluene Trifluorotoluene is an organic compound with the formula of C6H5CF3. This colorless fluorocarbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For exam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value. The mixture is referred to as both xylene and, more precisely, xylenes. Mixed xylenes refers to a mixture of the xylenes plus ethylbenzene. The four compounds have identical molecular formulas . Typically the four compounds are produced together by various catalytic reforming and pyrolysis methods. Occurrence and production Xylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. They also occur in crude oil in concentrations of about 0.5–1%, depending on the source. Small quantities occur in gasoline and aircra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radical Halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of UV light. The reaction is used for the industrial synthesis of chloroform (CHCl3), dichloromethane (CH2Cl2), and hexachlorobutadiene. It proceeds by a free-radical chain mechanism. General mechanism The chain mechanism is as follows, using the chlorination of methane as an example: ; Initiation: Ultraviolet radiation splits ( homolyzes) a chlorine molecule to two chlorine atom radicals. ; Chain propagation (two steps): A radical abstracts a hydrogen atom from methane, leaving a primary methyl radical. The methyl radical then abstracts Cl• from Cl2 to give the desired product and another chlorine radical. The radical will then participate in another propagation reaction: the radical chain. Other products such as CH2Cl2 may also form. ; Chain termination: Two free radicals (chlorine and chlorine, chlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KMnO4
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, which dissolves in water as K+ and ions to give an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is commonly used as a biocide for water treatment purposes. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tons. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four ligands are bound to a manganese(VII) center. Structure forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allylic
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other reactions that tend to occur with al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
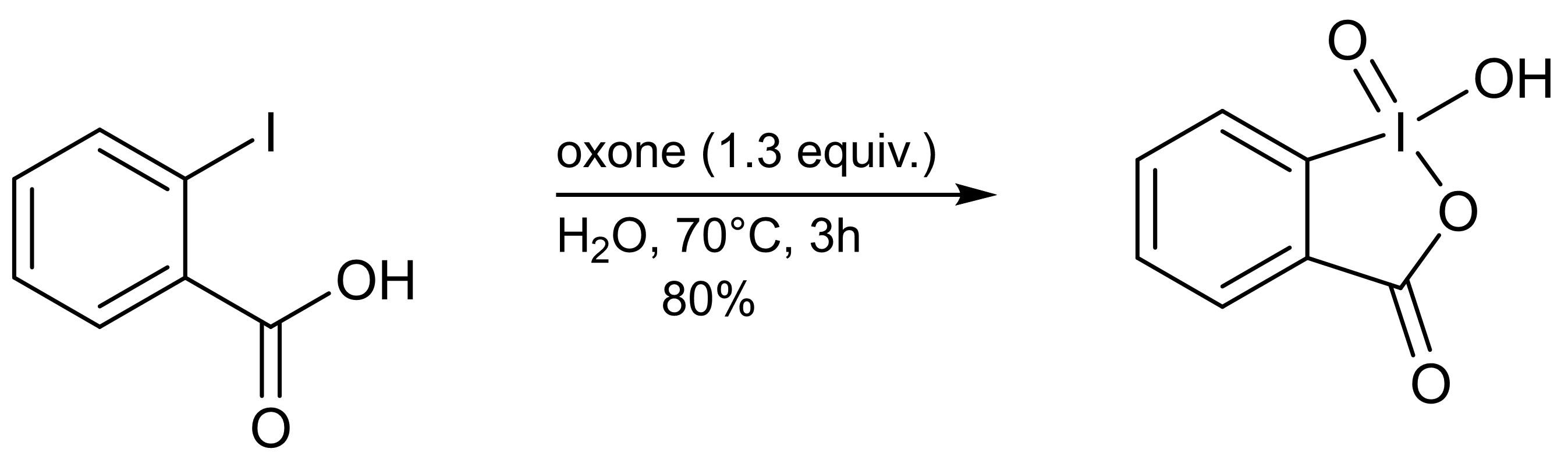
2-Iodoxybenzoic Acid
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is most often prepared from 2-iodobenzoic acid and a strong oxidant such as potassium bromate and sulfuric acid, or more commonly, oxone. One of the main drawbacks of IBX is its limited solubility; IBX is insoluble in many common organic solvents. IBX is an impact- and heat-sensitive explosive (>200°C). Commercial IBX is stabilized by carboxylic acids such as benzoic acid and isophthalic acid. Preparation IBX can be prepared in a single step by adding an excess of oxone to an aqueous solution of 2-iodobenzoic acid. After warming the solution to 70°C for three hours, the precipitated IBX is collected as a white crystalline solid (80% yield, ≥95% purity). Decomposition of IBX to 2-iodosobenzoic acid and 2-iodobenzoic acid occurs at elevated temperatures, and therefore purification by recrystallization ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen atom, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with and is represented by the symbol Ph (archaically, Φ), or Ø. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzhydryl Compounds
The benzhydryl compounds are a group of organic compounds whose parent structures include diphenylmethane (which is two benzene rings connected by a single methane), with any number of attached substituents, including bridges. This group typically excludes compounds in which either benzene is fused to another ring (bicyclic, tricyclic, polycyclic) or includes a heteroatom, or where the methane connects to three or four benzenes. The benzhydryl '' radical'' can be abbreviated or Bzh. Carboaromatic Alcohols *''Acyclic:'' pridinol *''Pyrolidino:'' diphenylprolinol *''2-Piperidine:'' pipradrol *''4-Piperidine:'' terfenadine, fexofenadine *''Benzilic ester:'' QNB, JB-336, JB-318, benactyzine Alkenes *''Tricycle:'' amitriptyline, melitracen, cyclobenzaprine, tianeptine, amineptine, clopenthixol, chlorprothixene, flupentixol, thiothixene, zuclopenthixol *''Tricyclic and piperidine:'' pimethixene, cyproheptadine *''Acyclic:'' gilutensin Alkyl(amine)s *''Acyclic:'' (3- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,5-Dimethylpyrazole
3,5-Dimethylpyrazole is an organic compound with the formula (CH3C)2CHN2H. It is one of several isomeric derivatives of pyrazole that contain two methyl substituents. The compound is unsymmetrical but the corresponding conjugate acid (pyrazolium) and conjugate base (pyrazolide) have C2v symmetry. It is a white solid that dissolves well in polar organic solvents. It is a precursor to a variety of ligands that are widely studied in coordination chemistry including trispyrazolylborate, a trispyrazolylmethane, and a pyrazolyldiphosphine. Condensation of acetylacetone and hydrazine Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ... gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3C)2CHN2H + 2 H2O It has found use as a blocki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium Trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula . It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen. Production, structure, and basic reactions Chromium trioxide is generated by treating sodium dichromate with sulfuric acid: : Approximately 100,000 tonnes are produced annually by this or similar routes. The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3. The structure of monomeric has been ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |