Chromium trioxide on:
[Wikipedia]
[Google]
[Amazon]
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an
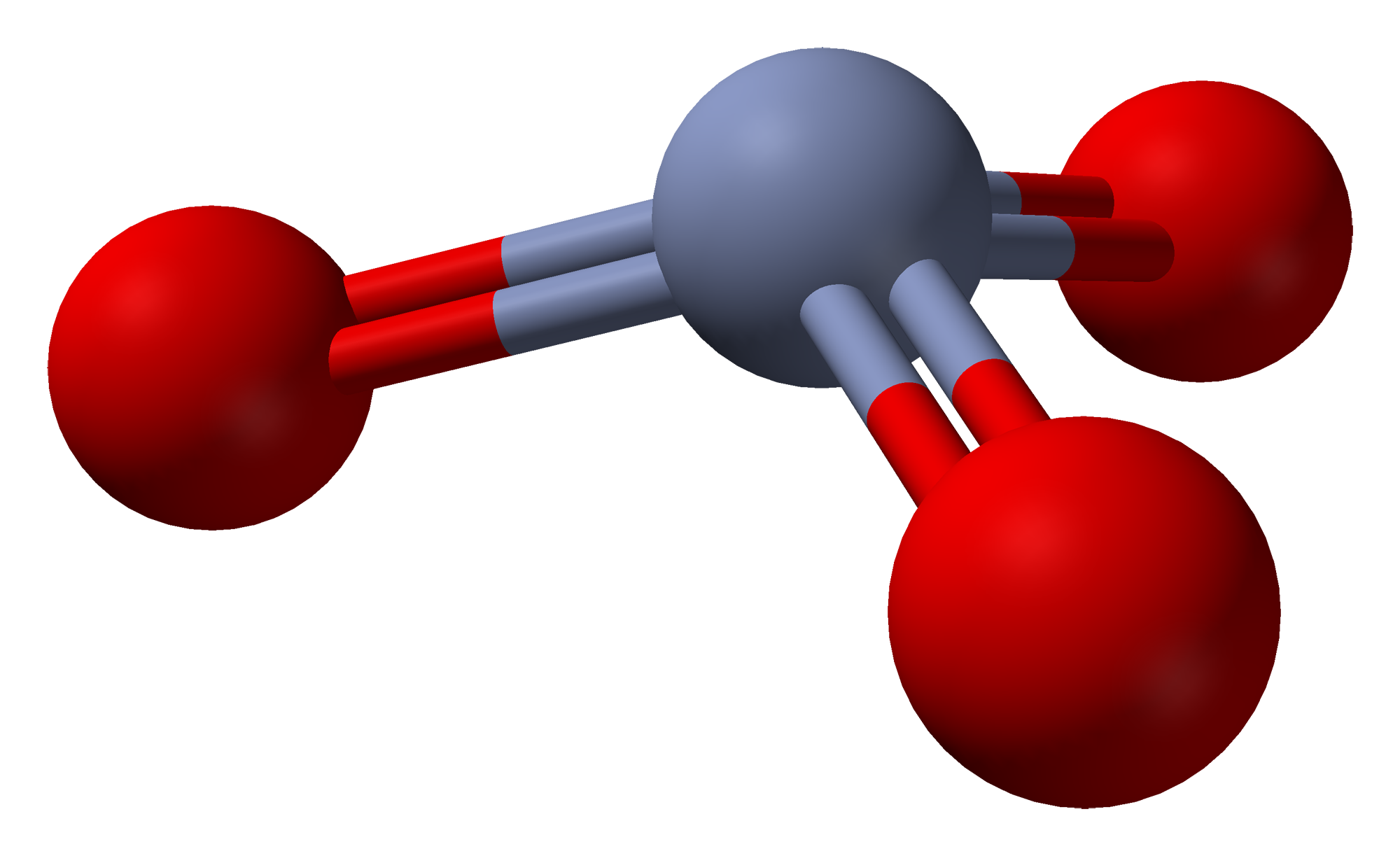
 The structure of monomeric has been calculated using density functional theory, and is predicted to be pyramidal ( point group C3v) rather than planar (point group D3h).
:
The structure of monomeric has been calculated using density functional theory, and is predicted to be pyramidal ( point group C3v) rather than planar (point group D3h).
: Chromium trioxide decomposes above 197 °C, liberating oxygen and eventually giving :
:
It is used in
Chromium trioxide decomposes above 197 °C, liberating oxygen and eventually giving :
:
It is used in
Reaction between potassium dichromate and sulfuric acid (1).jpg, A concentrated solution of potassium dichromate in water.
Reaction between potassium dichromate and sulfuric acid (2).jpg, Addition of sulfuric acid to the solution.
Reaction between potassium dichromate and sulfuric acid (3).jpg, Crystallization of chromium trioxide from the reaction.
Reaction between chromium(VI) oxide and ethanol (1).JPG, Reaction between chromium trioxide and ethanol
Reaction between chromium(VI) oxide and ethanol (2).JPG
Reaction between chromium(VI) oxide and ethanol (3).JPG
ATSDR Case Studies in Environmental Medicine: Chromium Toxicity
U.S.
Chromium Trioxide
at ''
Reactions with Chromium Trioxide as Oxidizing Agent
{{oxygen compounds Acidic oxides Chromium(VI) compounds
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.
This compound is a dark-purple solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the redox, reduction of cations of that metal by means of a direct current, direct electric cur ...
. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruse ...
.
Production, structure, and basic reactions
Chromium trioxide is generated by treating sodium dichromate withsulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
:
:
Approximately 100,000 tonnes are produced annually by this or similar routes.
The solid consists of chains of tetrahedrally coordinated chromium atoms that share vertices. Each chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
center therefore shares two oxygen centers with neighbors. Two oxygen atoms are not shared, giving an overall stoichiometry of 1:3.  The structure of monomeric has been calculated using density functional theory, and is predicted to be pyramidal ( point group C3v) rather than planar (point group D3h).
:
The structure of monomeric has been calculated using density functional theory, and is predicted to be pyramidal ( point group C3v) rather than planar (point group D3h).
: Chromium trioxide decomposes above 197 °C, liberating oxygen and eventually giving :
:
It is used in
Chromium trioxide decomposes above 197 °C, liberating oxygen and eventually giving :
:
It is used in organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
as an oxidant, often as a solution in acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, or acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
in the case of the Jones oxidation. In these oxidations, the Cr(VI) converts primary alcohols to the corresponding carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
s and secondary alcohols to ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s. The reactions are shown below:
* Primary alcohols to carboxylic acids
*:
* Secondary alcohols to ketones
*:
Applications
Chromium trioxide is mainly used in chrome plating. It is typically employed with additives that affect the plating process but do not react with the trioxide. The trioxide reacts withcadmium
Cadmium is a chemical element; it has chemical symbol, symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12 element, group 12, zinc and mercury (element), mercury. Like z ...
, zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
, and other metals to generate passivating chromate films that resist corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
. It is also used in the production of synthetic rubies. Chromic acid solution is also used in applying types of anodic coating to aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, which are primarily used in aerospace applications. On the International Space Station, it is used to control bacteria growth in the wastewater storage tank. A chromic acid/phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
solution is also the preferred stripping agent of anodic coatings of all types.
Safety
Chromium trioxide is highly toxic, corrosive, and carcinogenic. It is the main example ofhexavalent chromium
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is any chemical compound that contains the element chromium in the +6 oxidation state (thus hexavalent). It has been identified as carcinogenic, which is of concern since approximately of ...
, an environmental hazard.The environmental impact of hexavalent chromium inspired the 2000 biographical Hollywood movie '' Erin Brockovich''. The related chromium(III) derivatives are not particularly dangerous; thus, reductants are used to destroy chromium(VI) samples.
Chromium trioxide, being a powerful oxidizer, will ignite organic materials such as alcohols on contact.
Images
References
External links
ATSDR Case Studies in Environmental Medicine: Chromium Toxicity
U.S.
Department of Health and Human Services
The United States Department of Health and Human Services (HHS) is a cabinet-level executive branch department of the US federal government created to protect the health of the US people and providing essential human services. Its motto is ...
Chromium Trioxide
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
Reactions with Chromium Trioxide as Oxidizing Agent
{{oxygen compounds Acidic oxides Chromium(VI) compounds