|
Tantalum(V) Chloride
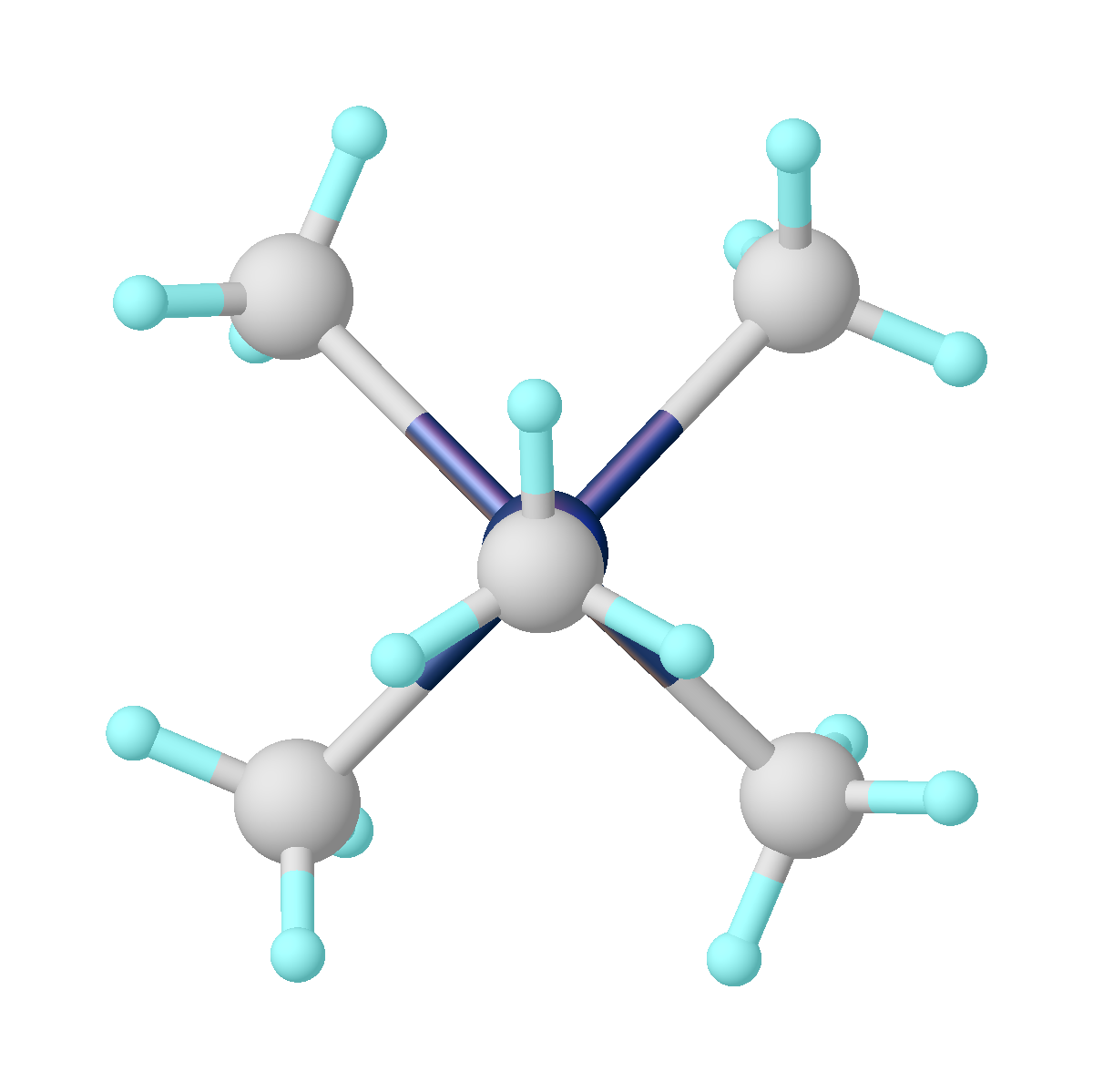
Tantalum(V) chloride, also known as tantalum pentachloride, is an inorganic compound with the formula . It takes the form of a white powder, and is commonly used as a starting material in tantalum chemistry. It readily hydrolyzes to form tantalum(V) oxytrichloride () and eventually tantalum pentoxide (); this requires that it be synthesised and manipulated under anhydrous conditions, using air-free techniques. Structure crystallizes in the monoclinic space group ''C''2/''m''. The ten chlorine atoms define a pair of octahedra that share a common edge. The tantalum atoms occupy the centres of the octahedra and are joined by two chlorine bridging ligands. The dimeric structure is retained in non-complexing solvents and to a large extent in the molten state. In the vapour state, however, is monomeric. This monomer adopts a trigonal bipyramidal structure, like that of . Physical Properties The solubility of tantalum pentachloride increases slightly for the following series of ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LEAD
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its Amphoterism, amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Lead compounds, Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition metal that is highly corrosion-resistant. It is part of the refractory metals group, which are widely used as components of strong superalloy, high-melting-point alloys. It is a group 5 element, along with vanadium and niobium, and it always occurs in geologic sources together with the chemically similar niobium, mainly in the mineral groups tantalite, columbite, and coltan. The chemical inertness and very high melting point of tantalum make it valuable for laboratory and industrial equipment such as Chemical reactor, reaction vessels and vacuum furnaces. It is used in tantalum capacitors for electronic equipment such as computers. It is being investigated for use as a material for high-quality superconducting resonators in quantum proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC nomenclature of organic chemistry, IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Hydrocarbon
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's rule. Aromatic compounds have the following general properties: * Typically unreactive * Often non polar and hydrophobic * High carbon-hydrogen ratio * Burn with a strong sooty yellow flame, due to high C:H ratio * Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride. Structure The structures for the phosphorus chlorides are invariably consistent with VSEPR theory. The structure of depends on its environment. Gaseous and molten is a neutral molecule with trigonal bipyramidal geometry and (''D''3h) symmetry. The hypervalent nature of this species (as well as of , see below) can be explained with the inclusion of non-bonding molecular orbitals (molecular orbital theory) or resonance (valence bond theory). This trigonal bipyramidal structure persists in nonpolar solvents, such as and . In the solid state is an ionic compound called tetrachlorophosphonium hexachlorophosphate formulated . In solutions of polar solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
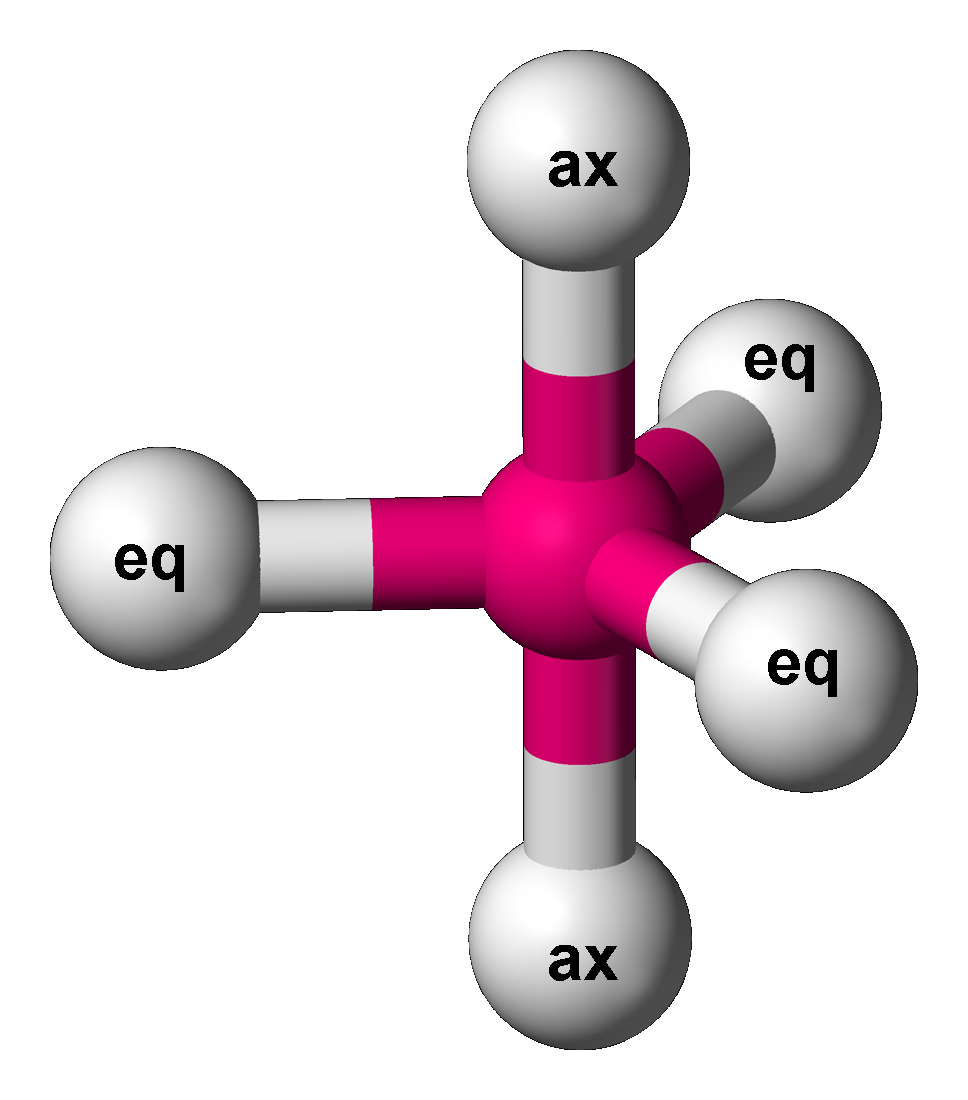
Trigonal Bipyramidal Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more cro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemistry classifies monomers by type, and two broad classes based on the type of polymer they form. By type: * natural vs synthetic, e.g. glycine vs caprolactam, respectively * polar vs nonpolar, e.g. vinyl acetate vs ethylene, respectively * cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively By type of polymer they form: * those that participate in condensation polymerization * those that participate in addition polymerization Differing stoichiometry causes each class to create its respective form of polymer. : The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of a repeating pattern in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of the pattern that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Air-free Technique
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen. The two most common types of air-free technique involve the use of a glovebox and a Schlenk line, although some rigorous applications use a high-vacuum line. In both methods, glassware (often Schlenk tubes) are pre-dried in ovens prior to use. They may be flame-dried to remove adsorbed water. Prior to coming into an inert atmosphere, vessels are further dried by ''purge-and-refill'' — the vessel is subjected to a vacuum to remove gases and water, and then refilled with inert gas. This cycle is usually ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achieve perfect dryness; anhydrous compounds gradually absorb water from the atmosphere so they must be stored carefully. Solids Many salts and solids can be dried using heat, or under vacuum. Desiccators can also be used to store reagents in dry conditions. Common desiccants include phosphorus pentoxide and silica gel. Chemists may also require dry glassware for sensitive reactions. This can be achieved by drying glassware in an oven, by flame, or under vacuum. Dry solids can be produced by freeze-drying, which is also known as lyophilization. Liquids or solvents In many cases, the presence of water can prevent a reaction from happening, or cause undesirable products to form. To prevent this, anhydrous solvents must be used when perform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |