trigonal bipyramidal molecular geometry on:
[Wikipedia]
[Google]
[Amazon]
In
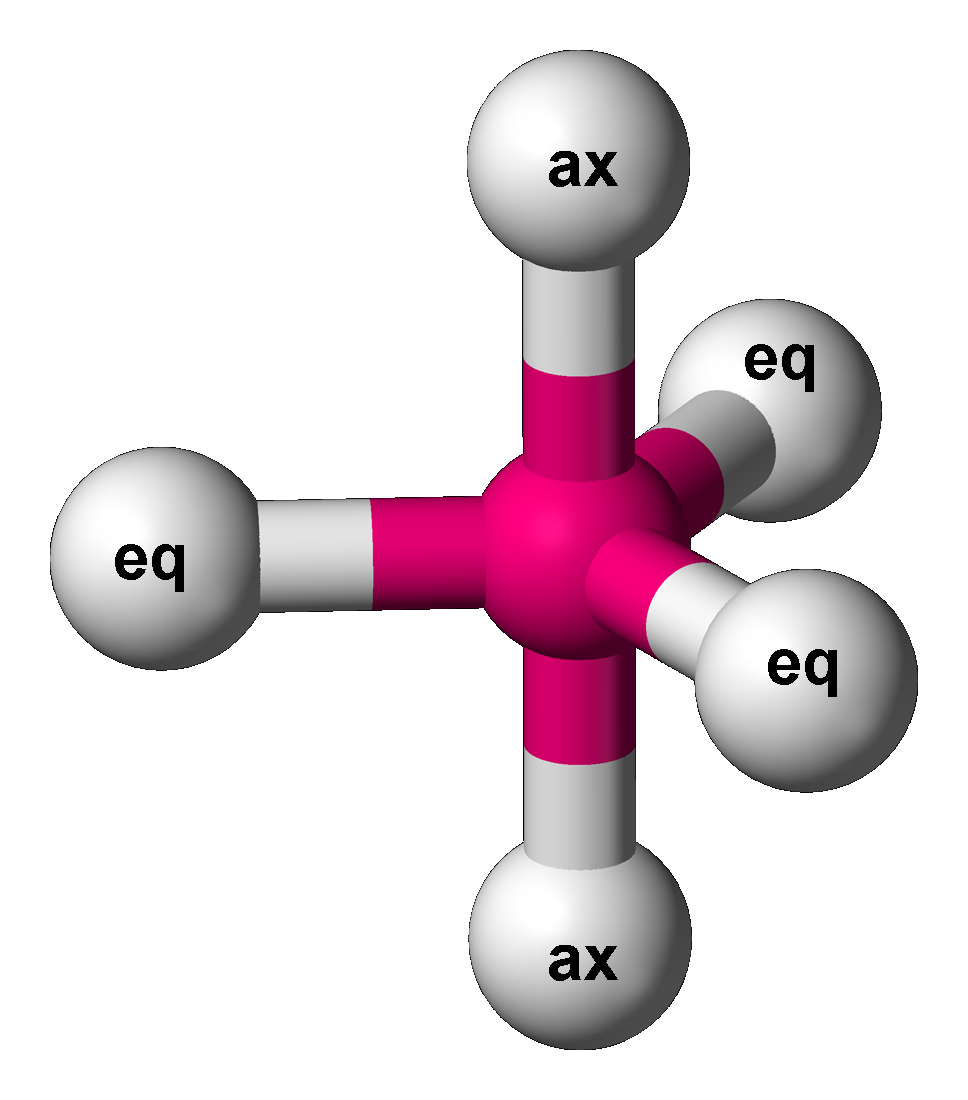 The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For
The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For
Indiana University Molecular Structure Center
{{MolecularGeometry Stereochemistry Molecular geometry
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, a trigonal bipyramid formation is a molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid
The pentagonal bipyramid (or pentagonal dipyramid) is a polyhedron with ten triangular faces. It is constructed by attaching two pentagonal pyramids to each of their bases. If the triangular faces are equilateral, the pentagonal bipyramid is an ...
), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, althoug ...
() in the gas phase.
Axial (or apical) and equatorial positions
 The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For
The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, althoug ...
as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions).
According to the VSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ) is a conceptual model, model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gill ...
of molecular geometry, an axial position is more crowded because an axial atom has three neighboring equatorial atoms (on the same central atom) at a 90° bond angle, whereas an equatorial atom has only two neighboring axial atoms at a 90° bond angle. For molecules with five identical ligands, the axial bond lengths tend to be longer because the ligand atom cannot approach the central atom as closely. As examples, in PF5 the axial P−F bond length is 158 pm and the equatorial is 152 pm, and in PCl5 the axial and equatorial are 214 and 202 pm respectively.
In the mixed halide PF3Cl2 the chlorines occupy two of the equatorial positions, indicating that fluorine has a greater apicophilicity or tendency to occupy an axial position. In general ligand apicophilicity increases with electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
and also with pi-electron withdrawing ability, as in the sequence Cl < F < CN. Both factors decrease electron density in the bonding region near the central atom so that crowding in the axial position is less important.
Related geometries with lone pairs
The VSEPR theory also predicts that substitution of a ligand at a central atom by a lone pair of valence electrons leaves the general form of the electron arrangement unchanged with the lone pair now occupying one position. For molecules with five pairs of valence electrons including both bonding pairs and lone pairs, the electron pairs are still arranged in a trigonal bipyramid but one or more equatorial positions is not attached to a ligand atom so that the molecular geometry (for the nuclei only) is different. The seesaw molecular geometry is found in sulfur tetrafluoride (SF4) with a central sulfur atom surrounded by four fluorine atoms occupying two axial and two equatorial positions, as well as one equatorial lone pair, corresponding to an AX4E molecule in the AXE notation. AT-shaped molecular geometry
In chemistry, T-shaped molecular geometry describes the structures of some molecules where a central atom has three ligands. Ordinarily, three-coordinated compounds adopt Trigonal planar molecular geometry, trigonal planar or Trigonal pyramidal m ...
is found in chlorine trifluoride (ClF3), an AX3E2 molecule with fluorine atoms in two axial and one equatorial position, as well as two equatorial lone pairs. Finally, the triiodide ion () is also based upon a trigonal bipyramid, but the actual molecular geometry is linear
In mathematics, the term ''linear'' is used in two distinct senses for two different properties:
* linearity of a '' function'' (or '' mapping'');
* linearity of a '' polynomial''.
An example of a linear function is the function defined by f(x) ...
with terminal iodine atoms in the two axial positions only and the three equatorial positions occupied by lone pairs of electrons (AX2E3); another example of this geometry is provided by xenon difluoride, XeF2.
Berry pseudorotation
Isomers with a trigonal bipyramidal geometry are able to interconvert through a process known as Berry pseudorotation. Pseudorotation is similar in concept to the movement of a conformational diastereomer, though no full revolutions are completed. In the process of pseudorotation, two equatorial ligands (both of which have a shorter bond length than the third) "shift" toward the molecule's axis, while the axial ligands simultaneously "shift" toward the equator, creating a constant cyclical movement. Pseudorotation is particularly notable in simple molecules such as phosphorus pentafluoride (PF5).See also
* AXE method *Molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
References
External links
Indiana University Molecular Structure Center
{{MolecularGeometry Stereochemistry Molecular geometry