Lead () is a
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has
symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Pb (from
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
) and
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
82. It is a
heavy metal that is
denser than most common materials. Lead is
soft and
malleable
Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversi ...
, and also has a relatively low
melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
. When freshly cut, lead is a shiny gray with a hint of blue. It
tarnish
Tarnish is a thin layer of corrosion that forms over copper, brass, aluminum, magnesium, neodymium and other similar metals as their outermost layer undergoes a chemical reaction. Tarnish does not always result from the sole effects of oxygen in ...
es to a dull gray color when exposed to air. Lead has the highest atomic number of any
stable element and three of its
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s are endpoints of major nuclear
decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
s of heavier elements.
Lead is a relatively unreactive
post-transition metal
The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metal ...
. Its weak metallic character is illustrated by its
amphoteric nature; lead and
lead oxide
Lead oxides are a group of inorganic compounds with formulas including lead (Pb) and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), gr ...
s react with
acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
s and
bases, and it tends to form
covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
s.
Compounds of lead are usually found in the +2
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
rather than the +4 state common with lighter members of the
carbon group
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Ch ...
. Exceptions are mostly limited to
organolead compounds. Like the lighter members of the group, lead tends to
bond with itself; it can form chains and polyhedral structures.
Since lead is easily extracted from its
ores, prehistoric people in the Near East
were aware of it.
Galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crysta ...
is a principal ore of lead which often bears silver. Interest in silver helped initiate widespread extraction and use of lead in
ancient Rome
In modern historiography, ancient Rome is the Roman people, Roman civilisation from the founding of Rome, founding of the Italian city of Rome in the 8th century BC to the Fall of the Western Roman Empire, collapse of the Western Roman Em ...
. Lead production declined after the
fall of Rome
The fall of the Western Roman Empire, also called the fall of the Roman Empire or the fall of Rome, was the loss of central political control in the Western Roman Empire, a process in which the Empire failed to enforce its rule, and its vast ...
and did not reach comparable levels until the
Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
. Lead played a crucial role in the development of the
printing press
A printing press is a mechanical device for applying pressure to an inked surface resting upon a printing, print medium (such as paper or cloth), thereby transferring the ink. It marked a dramatic improvement on earlier printing methods in whi ...
, as
movable type
Movable type (US English; moveable type in British English) is the system and technology of printing and typography that uses movable Sort (typesetting), components to reproduce the elements of a document (usually individual alphanumeric charac ...
could be relatively easily cast from lead alloys. In 2014, the annual global production of lead was about ten million tonnes, over half of which was from recycling. Lead's high density, low melting point,
ductility
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic def ...
and relative inertness to
oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
make it useful. These properties, combined with its relative abundance and low cost, resulted in its extensive use in
construction
Construction are processes involved in delivering buildings, infrastructure, industrial facilities, and associated activities through to the end of their life. It typically starts with planning, financing, and design that continues until the a ...
,
plumbing
Plumbing is any system that conveys fluids for a wide range of applications. Plumbing uses piping, pipes, valves, piping and plumbing fitting, plumbing fixtures, Storage tank, tanks, and other apparatuses to convey fluids. HVAC, Heating and co ...
,
batteries,
bullets
A bullet is a Kinetic energy weapon, kinetic projectile, a component of firearm ammunition that is Shooting, shot from a gun barrel. They are made of a variety of materials, such as copper, lead, steel, polymer, rubber and even wax; and are made ...
,
shots,
weights,
solder
Solder (; North American English, NA: ) is a fusible alloy, fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces aft ...
s,
pewters,
fusible alloy
A fusible alloy is a metal alloy capable of being easily fused, i.e. easily meltable, at relatively low temperatures. Fusible alloys are commonly, but not necessarily, eutectic alloys.
Sometimes the term "fusible alloy" is used to describe alloy ...
s,
lead paint
Lead paint or lead-based paint is paint containing lead. As pigment, lead(II) chromate (, "chrome yellow"), lead(II,IV) oxide, (, "red lead"), and lead(II) carbonate (, "white lead") are the most common forms.. Lead is added to paint to acceler ...
s,
leaded gasoline, and
radiation shielding.
Lead is a
neurotoxin
Neurotoxins are toxins that are destructive to nervous tissue, nerve tissue (causing neurotoxicity). Neurotoxins are an extensive class of exogenous chemical neurological insult (medical), insultsSpencer 2000 that can adversely affect function ...
that accumulates in soft tissues and bones. It damages the nervous system and interferes with the function of biological
enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s, causing
neurological disorder
Neurological disorders represent a complex array of medical conditions that fundamentally disrupt the functioning of the nervous system. These disorders affect the brain, spinal cord, and nerve networks, presenting unique diagnosis, treatment, and ...
s ranging from behavioral problems to brain damage, and also affects general health, cardiovascular, and renal systems.
Lead's toxicity was first documented by ancient Greek and Roman writers, who noted some of the symptoms of
lead poisoning
Lead poisoning, also known as plumbism and saturnism, is a type of metal poisoning caused by lead in the body. Symptoms may include abdominal pain, constipation, headaches, irritability, memory problems, infertility, numbness and paresthesia, t ...
, but became widely recognized in Europe in the late 19th century.
Physical properties
Atomic
A lead
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
has 82
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, arranged in an
electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
of [
Xe]4f
145d
106s
26p
2. The sum of lead's first and second
ionization energies—the total energy required to remove the two 6p electrons—is close to that of
tin, lead's upper neighbor in the
carbon group
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Ch ...
. This is unusual; ionization energies generally fall going down a group, as an element's outer electrons become more distant from the
nucleus, and more
shielded by smaller orbitals. The sum of the first four ionization energies of lead exceeds that of tin, contrary to what
periodic trends
In chemistry, periodic trends are specific patterns present in the periodic table that illustrate different aspects of certain Chemical element, elements when grouped by period (periodic table), period and/or Group (periodic table), group. They w ...
would predict. This is explained by
relativistic effects, which become significant in heavier atoms, which contract s and p orbitals such that lead's 6s electrons have larger binding energies than its 5s electrons. A consequence is the so-called
inert pair effect
The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of o ...
: the 6s electrons of lead become reluctant to participate in bonding, stabilising the +2
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
and making the distance between nearest atoms in
crystalline
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macrosc ...
lead unusually long.
Lead's lighter carbon group
congeners form stable or metastable
allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
with the tetrahedrally coordinated and
covalently bonded diamond cubic
In crystallography, the diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group 14 also adopt this structure, in ...
structure. The energy levels of their outer
s- and
p-orbitals are close enough to allow mixing into four
hybrid sp
3 orbitals. In lead, the inert pair effect increases the separation between its s- and p-orbitals, and the gap cannot be overcome by the energy that would be released by extra bonds following hybridization. Rather than having a diamond cubic structure, lead forms
metallic bonds in which only the p-electrons are delocalized and shared between the Pb
2+ ions. Lead consequently has a
face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
structure like the similarly sized
divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
metals
calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
and
strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
.
Bulk
Pure lead has a bright, shiny gray appearance with a hint of blue. It tarnishes on contact with moist air and takes on a dull appearance, the hue of which depends on the prevailing conditions. Characteristic properties of lead include high
density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be u ...
, malleability, ductility, and high resistance to
corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
due to
passivation.
Lead's close-packed face-centered cubic structure and high atomic weight result in a density of 11.34 g/cm
3, which is greater than that of common metals such as
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
(7.87 g/cm
3),
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
(8.93 g/cm
3), and
zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
(7.14 g/cm
3). This density is the origin of the idiom ''to go over like a lead balloon''. Some rarer metals are denser:
tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
and
gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
are both at 19.3 g/cm
3, and
osmium—the densest metal known—has a density of 22.59 g/cm
3, almost twice that of lead.
Lead is a very soft metal with a
Mohs hardness
The Mohs scale ( ) of mineral hardness is a qualitative ordinal scale, from 1 to 10, characterizing scratch resistance of mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fair ...
of 1.5; it can be scratched with a fingernail. It is quite malleable and somewhat ductile. The
bulk modulus
The bulk modulus (K or B or k) of a substance is a measure of the resistance of a substance to bulk compression. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume.
Other mo ...
of lead—a measure of its ease of compressibility—is 45.8
GPa. In comparison, that of
aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
is 75.2 GPa; copper 137.8 GPa; and
mild steel 160–169 GPa. Lead's
tensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
, at 12–17 MPa, is low (that of aluminium is 6 times higher, copper 10 times, and mild steel 15 times higher); it can be strengthened by adding small amounts of copper or
antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
.

The melting point of lead—at 327.5 °C (621.5 °F)—is very low compared to most metals. Its
boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of 1749 °C (3180 °F) is the lowest among the carbon-group elements. The
electrical resistivity
Electricity is the set of physical phenomena associated with the presence and motion of matter possessing an electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
of lead at 20 °C is 192
nanoohm-meters, almost an
order of magnitude
In a ratio scale based on powers of ten, the order of magnitude is a measure of the nearness of two figures. Two numbers are "within an order of magnitude" of each other if their ratio is between 1/10 and 10. In other words, the two numbers are ...
higher than those of other industrial metals (copper at ; gold ; and aluminium at ). Lead is a
superconductor at temperatures lower than 7.19
K; this is the highest
critical temperature
Critical or Critically may refer to:
*Critical, or critical but stable, medical states
**Critical, or intensive care medicine
*Critical juncture, a discontinuous change studied in the social sciences.
*Critical Software, a company specializing in ...
of all
type-I superconductors and the third highest of the elemental superconductors.
Isotopes
Natural lead consists of four stable
isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s with mass numbers of 204, 206, 207, and 208, and traces of six short-lived radioisotopes with mass numbers 209–214 inclusive. The high number of isotopes is consistent with lead's
atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
being even. Lead has a
magic number of protons (82), for which the
nuclear shell model
In nuclear physics, atomic physics, and nuclear chemistry, the nuclear shell model utilizes the Pauli exclusion principle to model the structure of atomic nuclei in terms of energy levels. The first shell model was proposed by Dmitri Ivanenk ...
accurately predicts an especially stable nucleus. Lead-208 has 126 neutrons, another magic number, which may explain why lead-208 is extraordinarily stable.
With its high atomic number, lead is the heaviest element whose natural isotopes are regarded as stable; lead-208 is the heaviest stable nucleus. (This distinction formerly fell to
bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
, with an atomic number of 83, until its only
primordial isotope
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
, bismuth-209, was found in 2003 to decay very slowly.) The four stable isotopes of lead could theoretically undergo
alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus). The parent nucleus transforms or "decays" into a daughter product, with a mass number that is reduced by four and an a ...
to isotopes of
mercury with a release of energy, but this has not been observed for any of them; their predicted half-lives range from 10
35 to 10
189 years (at least 10
25 times the current age of the universe).

Three of the stable isotopes are found in three of the four major
decay chain
In nuclear science a decay chain refers to the predictable series of radioactive disintegrations undergone by the nuclei of certain unstable chemical elements.
Radioactive isotopes do not usually decay directly to stable isotopes, but rather ...
s: lead-206, lead-207, and lead-208 are the final decay products of
uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it i ...
,
uranium-235
Uranium-235 ( or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exists in nat ...
, and
thorium-232
Thorium-232 () is the main naturally occurring isotope of thorium, with a relative abundance of 99.98%. It has a half life of 14.05 billion years, which makes it the longest-lived isotope of thorium. It decays by alpha decay to radium-228; its de ...
, respectively. These decay chains are called the
uranium chain, the
actinium chain, and the
thorium chain. Their isotopic concentrations in a natural rock sample depends greatly on the presence of these three parent uranium and thorium isotopes. For example, the relative abundance of lead-208 can range from 52% in normal samples to 90% in thorium ores; for this reason, the standard atomic weight of lead is given to only one decimal place. As time passes, the ratio of lead-206 and lead-207 to lead-204 increases, since the former two are supplemented by radioactive decay of heavier elements while the latter is not; this allows for
lead–lead dating. As uranium decays into lead, their relative amounts change; this is the basis for
uranium–lead dating
Uranium–lead dating, abbreviated U–Pb dating, is one of the oldest and most refined of the radiometric dating schemes. It can be used to date rocks that formed and crystallised from about 1 million years to over 4.5 billion years ago with routi ...
. Lead-207 exhibits
nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
, a property that has been used to study its compounds in solution and solid state, including in the human body.
Apart from the stable isotopes, which make up almost all lead that exists naturally, there are
trace quantities of a few radioactive isotopes. One of them is lead-210; although it has a half-life of only 22.2 years, small quantities occur in nature because lead-210 is produced by a long decay series that starts with uranium-238 (that has been present for billions of years on Earth). Lead-211, −212, and −214 are present in the decay chains of uranium-235, thorium-232, and uranium-238, respectively, so traces of all three of these lead isotopes are found naturally. Minute traces of lead-209 arise from the very rare
cluster decay
Cluster decay, also named heavy particle radioactivity, heavy ion radioactivity or heavy cluster decay," is a rare type of nuclear decay in which an atomic nucleus emits a small "cluster" of neutrons and protons, more than in an alpha particle, ...
of radium-223, one of the
daughter products of natural uranium-235, the rare beta-minus-neutron decay of thallium-210 (a decay product of uranium-238), and the decay chain of neptunium-237, traces of which are produced by
neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
in uranium ores. Lead-213 also occurs in the decay chain of neptunium-237. Lead-210 is particularly useful for helping to identify the ages of samples by measuring its ratio to lead-206 (both isotopes are present in a single decay chain).
In total, 43 lead isotopes have been synthesized, with mass numbers 178–220. Lead-205 is the most stable radioisotope, with a half-life of around 1.70 years. The second-most stable is lead-202, which has a half-life of about 52,500 years, longer than any of the natural trace radioisotopes.
Chemistry
Bulk lead exposed to moist air forms a protective layer of varying composition.
Lead(II) carbonate is a common constituent; the
sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
or
chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
may also be present in urban or maritime settings. This layer makes bulk lead effectively chemically inert in the air. Finely powdered lead, as with many metals, is
pyrophoric
A substance is pyrophoric (from , , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolithium compounds and triethylb ...
, and burns with a bluish-white flame.
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
reacts with lead at room temperature, forming
lead(II) fluoride. The reaction with
chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
is similar but requires heating, as the resulting chloride layer diminishes the reactivity of the elements. Molten lead reacts with the
chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the rad ...
s to give lead(II) chalcogenides.
Lead metal resists
sulfuric and
phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
but not
hydrochloric or
nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
; the outcome depends on insolubility and subsequent passivation of the product salt. Organic acids, such as
acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, dissolve lead in the presence of oxygen. Concentrated
alkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
s dissolve lead and form
plumbites.
Inorganic compounds
Lead shows two main oxidation states: +4 and +2. The
tetravalent state is common for the carbon group. The divalent state is rare for
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
and
silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
, minor for germanium, important (but not prevailing) for tin, and is the more important of the two oxidation states for lead. This is attributable to
relativistic effects, specifically the
inert pair effect
The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of o ...
, which manifests itself when there is a large difference in
electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
between lead and
oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
,
halide, or
nitride
In chemistry, a nitride is a chemical compound of nitrogen. Nitrides can be inorganic or organic, ionic or covalent. The nitride anion, N3−, is very elusive but compounds of nitride are numerous, although rarely naturally occurring. Some nitr ...
anions, leading to a significant partial positive charge on lead. The result is a stronger contraction of the lead 6s orbital than is the case for the 6p orbital, making it rather inert in ionic compounds. The inert pair effect is less applicable to compounds in which lead forms covalent bonds with elements of similar electronegativity, such as carbon in organolead compounds. In these, the 6s and 6p orbitals remain similarly sized and sp
3 hybridization is still energetically favorable. Lead, like carbon, is predominantly tetravalent in such compounds.
There is a relatively large difference in the electronegativity of lead(II) at 1.87 and lead(IV) at 2.33. This difference marks the reversal in the trend of increasing stability of the +4 oxidation state going down the carbon group; tin, by comparison, has values of 1.80 in the +2 oxidation state and 1.96 in the +4 state.
Lead(II)
Lead(II) compounds are characteristic of the inorganic chemistry of lead. Even strong
oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
s like fluorine and chlorine react with lead to give only
PbF2 and
PbCl2. Lead(II) ions are usually colorless in solution, and partially hydrolyze to form Pb(OH)
+ and finally
b4(OH)4sup>4+ (in which the
hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
ions act as
bridging ligands), but are not
reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
s as tin(II) ions are.
Techniques for identifying the presence of the Pb
2+ ion in water generally rely on the precipitation of lead(II) chloride using dilute hydrochloric acid. As the chloride salt is sparingly soluble in water, in very dilute solutions the precipitation of
lead(II) sulfide
Lead(II) sulfide (also spelled '' sulphide'') is an inorganic compound with the formula Pb S. Galena is the principal ore and the most important compound of lead. It is a semiconducting material with niche uses.
Formation, basic properties, rel ...
is instead achieved by bubbling
hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...
through the solution.
Lead monoxide exists in two
polymorphs,
litharge
Litharge (from Greek , 'stone' + 'silver' ) is one of the natural mineral forms of lead(II) oxide, PbO. Litharge is a secondary mineral which forms from the oxidation of galena ores. It forms as coatings and encrustations with internal tetr ...
α-PbO (red) and
massicot β-PbO (yellow), the latter being stable only above around 488 °C. Litharge is the most commonly used inorganic compound of lead. There is no lead(II) hydroxide; increasing the pH of solutions of lead(II) salts leads to hydrolysis and condensation. Lead commonly reacts with heavier chalcogens.
Lead sulfide is a
semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
, a
photoconductor, and an extremely sensitive
infrared radiation detector. The other two chalcogenides,
lead selenide and
lead telluride, are likewise photoconducting. They are unusual in that their color becomes lighter going down the group.
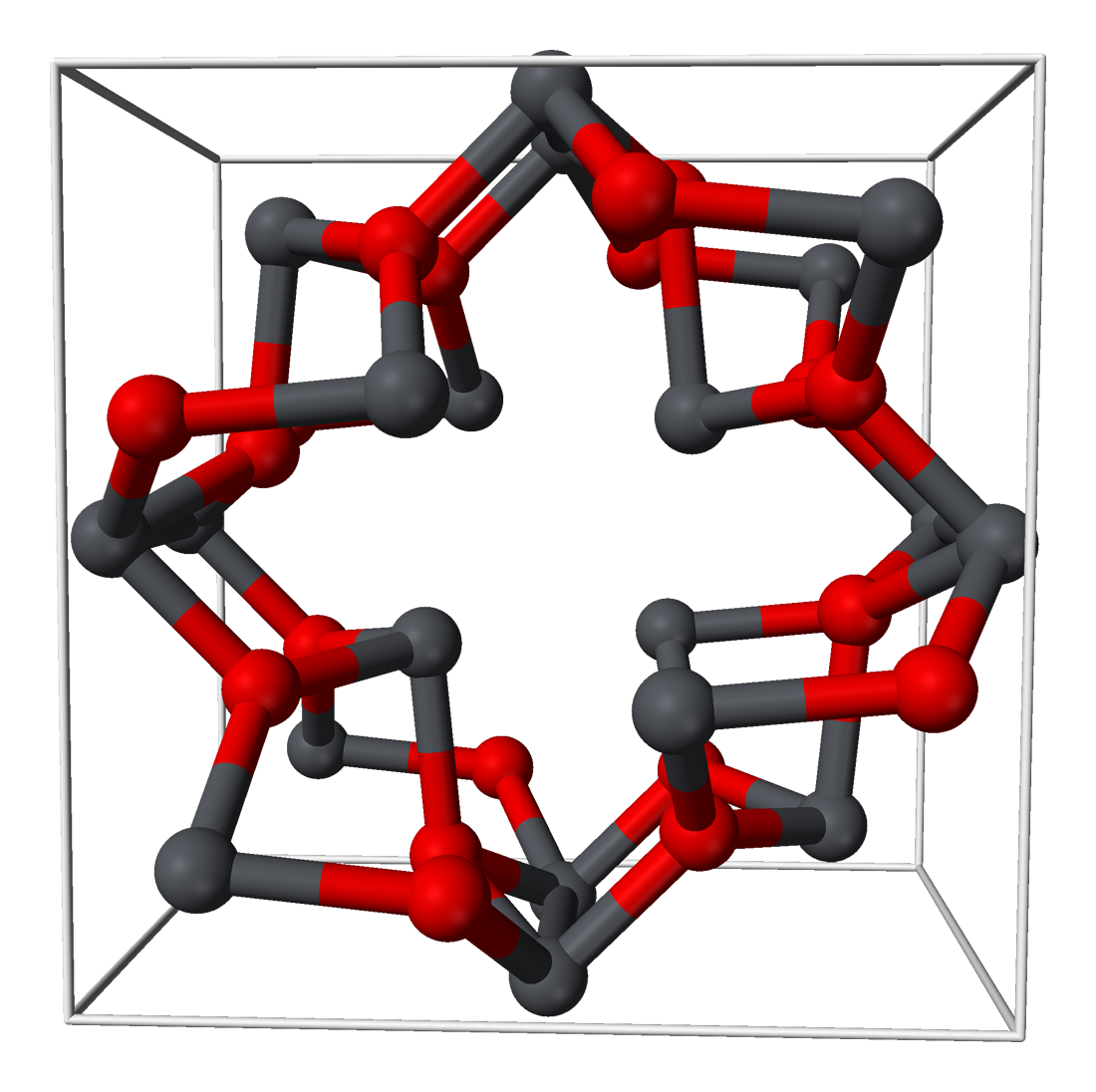
Lead dihalides are well-characterized; this includes the diastatide and mixed halides, such as PbFCl. The relative insolubility of the latter forms a useful basis for the
gravimetric determination of fluorine. The difluoride was the first solid
ionically conducting compound to be discovered (in 1834, by
Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic inducti ...
). The other dihalides decompose on exposure to ultraviolet or visible light, especially
the diiodide. Many lead(II)
pseudohalides are known, such as the cyanide, cyanate, and
thiocyanate. Lead(II) forms an extensive variety of halide
coordination complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of chemical bond, bound molecules or ions, that are in turn known as ' ...
es, such as
bCl4sup>2−,
bCl6sup>4−, and the
b2Cl9sub>''n''
5''n''− chain anion.
Lead(II) sulfate is insoluble in water, like the sulfates of other heavy divalent
cations.
Lead(II) nitrate and
lead(II) acetate are very soluble, and this is exploited in the synthesis of other lead compounds.
Lead(IV)
Few inorganic lead(IV) compounds are known. They are only formed in highly oxidizing solutions and do not normally exist under standard conditions. Lead(II) oxide gives a mixed oxide on further oxidation, Pb
3O
4. It is described as
lead(II,IV) oxide, or structurally 2PbO·PbO
2, and is the best-known mixed valence lead compound.
Lead dioxide is a strong oxidizing agent, capable of oxidizing hydrochloric acid to chlorine gas. This is because the expected PbCl
4 that would be produced is unstable and spontaneously decomposes to PbCl
2 and Cl
2. Analogously to
lead monoxide, lead dioxide is capable of forming
plumbate anions.
Lead disulfide and lead diselenide are only stable at high pressures.
Lead tetrafluoride, a yellow crystalline powder, is stable, but less so than the
difluoride.
Lead tetrachloride (a yellow oil) decomposes at room temperature, lead tetrabromide is less stable still, and the existence of lead tetraiodide is questionable.
Other oxidation states

Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained, as an intermediate between lead(II) and lead(IV), in larger organolead complexes; this oxidation state is not stable, as both the lead(III) ion and the larger complexes containing it are
radicals. The same applies for lead(I), which can be found in such radical species.
Numerous mixed lead(II,IV) oxides are known. When PbO
2 is heated in air, it becomes Pb
12O
19 at 293 °C, Pb
12O
17 at 351 °C, Pb
3O
4 at 374 °C, and finally PbO at 605 °C. A further
sesquioxide, Pb
2O
3, can be obtained at high pressure, along with several non-stoichiometric phases. Many of them show defective
fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scal ...
structures in which some oxygen atoms are replaced by vacancies: PbO can be considered as having such a structure, with every alternate layer of oxygen atoms absent.
Negative oxidation states can occur as
Zintl phases, as either free lead anions, as in Ba
2Pb, with lead formally being lead(−IV), or in oxygen-sensitive ring-shaped or polyhedral cluster ions such as the
trigonal bipyramidal Pb
52− ion, where two lead atoms are lead(−I) and three are lead(0). In such anions, each atom is at a polyhedral vertex and contributes two electrons to each covalent bond along an edge from their sp
3 hybrid orbitals, the other two being an external
lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
. They may be made in
liquid ammonia via the reduction of lead by
sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
.
Organolead

Lead can form
multiply-bonded chains, a property it shares with its lighter
homologs in the carbon group. Its capacity to do so is much less because the Pb–Pb
bond energy
In chemistry, bond energy (''BE'') is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase b ...
is over three and a half times lower than that of the
C–C bond. With itself, lead can build metal–metal bonds of an order up to three. With carbon, lead forms organolead compounds similar to, but generally less stable than, typical organic compounds (due to the Pb–C bond being rather weak). This makes the
organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
of lead far less wide-ranging than that of tin. Lead predominantly forms organolead(IV) compounds, even when starting with inorganic lead(II) reactants; very few organolead(II) compounds are known. The most well-characterized exceptions are Pb
H(SiMe3)2sub>2 and
plumbocene.
The lead analog of the simplest
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
,
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, is
plumbane
Plumbane is an inorganic chemical compound with the chemical formula PbH. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamicall ...
. Plumbane may be obtained in a reaction between metallic lead and atomic hydrogen. Two simple derivatives,
tetramethyllead and
tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
, are the best-known Organolead chemistry, organolead compounds. These compounds are relatively stable: tetraethyllead only starts to decompose if heated or if exposed to sunlight or ultraviolet light. With sodium metal, lead readily forms an equimolar alloy that reacts with Haloalkane, alkyl halides to form Organometallic chemistry, organometallic compounds such as tetraethyllead. The oxidizing nature of many organolead compounds is usefully exploited: Lead(IV) acetate, lead tetraacetate is an important laboratory reagent for oxidation in organic synthesis. Tetraethyllead, once added to automotive gasoline, was produced in larger quantities than any other organometallic compound, and is still widely used in avgas, fuel for small aircraft.
Other organolead compounds are less chemically stable. For many organic compounds, a lead analog does not exist.
Origin and occurrence
In space
Lead's per-particle abundance in the Solar System is 0.121 Parts-per notation, ppb (parts per billion). This figure is two and a half times higher than that of platinum, eight times more than Mercury (element), mercury, and seventeen times more than
gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
. The amount of lead in the universe is slowly increasing as most heavier atoms (all of which are unstable) gradually decay to lead. The abundance of lead in the Solar System since its formation 4.5 billion years ago has increased by about 0.75%. The Solar System abundances table shows that lead, despite its relatively high atomic number, is more prevalent than most other elements with atomic numbers greater than 40.
Primordial lead—which comprises the isotopes lead-204, lead-206, lead-207, and lead-208—was mostly created as a result of repetitive neutron capture processes occurring in stars. The two main modes of capture are the s-process, s- and r-processes.
In the s-process (s is for "slow"), captures are separated by years or decades, allowing less stable nuclei to undergo beta decay. A stable thallium-203 nucleus can capture a neutron and become thallium-204; this undergoes beta decay to give stable lead-204; on capturing another neutron, it becomes lead-205, which has a half-life of around 17 million years. Further captures result in lead-206, lead-207, and lead-208. On capturing another neutron, lead-208 becomes lead-209, which quickly decays into bismuth-209. On capturing another neutron, bismuth-209 becomes bismuth-210, and this beta decays to polonium-210, which alpha decays to lead-206. The cycle hence ends at lead-206, lead-207, lead-208, and bismuth-209.

In the r-process (r is for "rapid"), captures happen faster than nuclei can decay. This occurs in environments with a high neutron density, such as a supernova or the merger of two neutron stars. The neutron flux involved may be on the order of 10
22 neutrons per square centimeter per second. The r-process does not form as much lead as the s-process. It tends to stop once neutron-rich nuclei reach 126 neutrons. At this point, the neutrons are arranged in complete shells in the atomic nucleus, and it becomes harder to energetically accommodate more of them. When the neutron flux subsides, these nuclei beta decay into stable isotopes of
osmium, iridium, platinum.
On Earth
Lead is classified as a Goldschmidt classification#Chalcophile elements, chalcophile under the Goldschmidt classification, meaning it is generally found combined with sulfur. It rarely occurs in its native metal, native, metallic form. Many lead minerals are relatively light and, over the course of the Earth's history, have remained in the Earth's crust, crust instead of sinking deeper into the Earth's interior. This accounts for lead's relatively high Abundance of elements in Earth's crust, crustal abundance of 14 ppm; it is the 36th most Abundances of the elements (data page), abundant element in the crust.
The main lead-bearing mineral is galena (PbS), which is mostly found with zinc ores. Most other lead minerals are related to galena in some way; boulangerite, Pb
5Sb
4S
11, is a mixed sulfide derived from galena; anglesite, PbSO
4, is a product of galena oxidation; and cerussite or white lead ore, PbCO
3, is a Chemical decomposition, decomposition product of galena. Arsenic,
tin,
antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
, silver,
gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
,
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
,
bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
are common impurities in lead minerals.

World lead resources exceed two billion tons. Significant deposits are located in Australia, China, Ireland, Mexico, Peru, Portugal, Russia, United States. Global reserves—resources that are economically feasible to extract—totaled 88 million tons in 2016, of which Australia had 35 million, China 17 million, Russia 6.4 million.
Typical background concentrations of lead do not exceed 0.1 μg/m
3 in the atmosphere; 100 mg/kg in soil; 4 mg/kg in vegetation, 5 μg/L in fresh water and seawater.
Etymology
The modern English word ''lead'' is of Germanic origin; it comes from the Middle English and Old English (with the Macron (diacritic), macron above the "e" signifying that the vowel sound of that letter is long). The Old English word is derived from the hypothetical reconstructed Proto-Germanic language, Proto-Germanic ('lead'). According to linguistic theory, this word bore descendants in multiple Germanic languages of exactly the same meaning.
There is no consensus on the origin of the Proto-Germanic . One hypothesis suggests it is derived from Proto-Indo-European language, Proto-Indo-European ('lead'; capitalization of the vowel is equivalent to the macron). Another hypothesis suggests it is borrowed from Proto-Celtic language, Proto-Celtic ('lead'). This word is related to the
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
, which gave the element its chemical symbol ''Pb''. The word is thought to be the origin of Proto-Germanic (which also means 'lead'), from which stemmed the German .
The name of the chemical element is not related to the verb of the same spelling, which is derived from Proto-Germanic ('to lead').
History
Prehistory and early history

Metallic lead beads metals of antiquity, dating back to 7000–6500 BC have been found in Anatolia, Asia Minor and may represent the first example of metal smelting. At that time, lead had few (if any) applications due to its softness and dull appearance. The major reason for the spread of lead production was its association with silver, which may be obtained by burning galena (a common lead mineral). The Ancient Egyptians were the first to use lead minerals in cosmetics, an application that spread to Ancient Greece and beyond; the Egyptians had used lead for sinkers in Fishing net, fishing nets, Compacted oxide layer glaze, glazes, Glass, glasses, Vitreous enamel, enamels, Ornament (art), ornaments. Various civilizations of the Fertile Crescent used lead as a writing material, as coins, and as a List of building materials, construction material. Lead was used by the ancient Chinese as a stimulant, as currency, as Birth control, contraceptive, and in chopsticks. The Indus Valley Civilisation, Indus Valley civilization and the Mesoamericans used it for making Amulet, amulets; and the eastern and southern Africans used lead in wire drawing.
Classical era
Because silver was extensively used as a decorative material and an exchange medium, lead deposits came to be worked in Asia Minor from 3000 BC; later, lead deposits were developed in the Aegean Islands, Aegean and Lavrio, Laurion. These three regions collectively dominated production of mined lead until . Beginning c. 2000 BC, the Phoenicians worked deposits in the Iberian Peninsula, Iberian peninsula; by 1600 BC, lead mining existed in Cyprus, Greece, and Sardinia.

Roman Republic, Rome's territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the Classical antiquity, classical era, with an estimated annual output peaking at 80,000 tonnes. Like their predecessors, the Romans obtained lead mostly as a by-product of silver smelting. Lead mining occurred in central Europe, Roman Britain, Britain, Balkans, Greece, Anatolia, Hispania, the latter accounting for 40% of world production.

Lead tablets were commonly used as a material for letters. Lead coffins, cast in flat sand forms and with interchangeable motifs to suit the faith of the deceased, were used in ancient Judaea (Roman province), Judea. Lead was used to make sling bullets from the 5th century BC. In Roman times, lead sling bullets were amply used, and were effective at a distance of between 100 and 150 meters. The Balearic slinger, Balearic slingers, used as mercenaries in Carthaginian and Roman armies, were famous for their shooting distance and accuracy.
Lead was used for making water pipes in the Roman Empire; the
Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
word for the metal, , is the origin of the English word "
plumbing
Plumbing is any system that conveys fluids for a wide range of applications. Plumbing uses piping, pipes, valves, piping and plumbing fitting, plumbing fixtures, Storage tank, tanks, and other apparatuses to convey fluids. HVAC, Heating and co ...
". Its ease of working, its low melting point enabling the easy fabrication of completely waterproof welded joints, and its resistance to corrosion ensured its widespread use in other applications, including pharmaceuticals, roofing, currency, warfare. Writers of the time, such as Cato the Elder, Columella, and Pliny the Elder, recommended lead (and lead-coated) vessels for the preparation of Grape syrup, sweeteners and preservatives added to wine and food. The lead conferred an agreeable taste due to the formation of "sugar of lead" (
lead(II) acetate), whereas
copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
vessels imparted a bitter flavor through verdigris formation.
The Roman author Vitruvius reported the Roman lead poisoning theory, health dangers of lead and modern writers have suggested that lead poisoning played a major role in the Fall of the Western Roman Empire, decline of the Roman Empire. Other researchers have criticized such claims, pointing out, for instance, that not all abdominal pain is caused by lead poisoning. According to archaeological research, Roman Pipe (fluid conveyance), lead pipes increased lead levels in tap water but such an effect was "unlikely to have been truly harmful". When lead poisoning did occur, victims were called "saturnine", dark and cynical, after the ghoulish father of the gods, Saturn (mythology), Saturn. By association, lead was considered the father of all metals. Its status in Roman society was low as it was readily available and cheap.
Confusion with tin and antimony
Since the Bronze Age, metallurgists and engineers have understood the difference between rare and valuable
tin, essential for alloying with copper to produce tough and corrosion resistant bronze, and 'cheap and cheerful' lead. However, the nomenclature in some languages is similar. Romans called lead ("black lead"), and tin ("bright lead"). The association of lead and tin can be seen in other languages: the word in Czech language, Czech translates to "lead", but in Russian, its cognate () means "tin". To add to the confusion, lead bore a close relation to antimony: both elements commonly occur as sulfides (galena and stibnite), often together. Pliny incorrectly wrote that stibnite would give lead on heating, instead of antimony. In countries such as Turkey and India, the originally Persian name came to refer to either antimony sulfide or lead sulfide, and in some languages, such as Russian, gave its name to antimony ().
Middle Ages and the Renaissance
Lead mining in Western Europe declined after the fall of the Western Roman Empire, with Al-Andalus, Arabian Iberia being the only region having a significant output. The largest production of lead occurred in South Asia and East Asia, especially China and India, where lead mining grew rapidly.
In Europe, lead production began to increase in the 11th and 12th centuries, when it was again used for roofing and piping. Starting in the 13th century, lead was used to create medieval stained glass, stained glass. In the Alchemy#Medieval Europe, European and Alchemy in the medieval Islamic world, Arabian traditions of alchemy, lead (symbol ♄ in the European tradition) was considered an impure base metal which, by the separation, purification and balancing of its constituent essences, could be transformed to pure and incorruptible gold. During the period, lead was used increasingly for Adulterant, adulterating wine. The use of such wine was forbidden for use in Christian rites by a papal bull in 1498, but it continued to be imbibed and resulted in mass poisonings up to the late 18th century. Lead was a key material in parts of the
printing press
A printing press is a mechanical device for applying pressure to an inked surface resting upon a printing, print medium (such as paper or cloth), thereby transferring the ink. It marked a dramatic improvement on earlier printing methods in whi ...
, and lead dust was commonly inhaled by print workers, causing lead poisoning. Lead also became the chief material for making bullets for firearms: it was cheap, less damaging to iron gun barrels, had a higher density (which allowed for better retention of velocity), and its lower melting point made the production of bullets easier as they could be made using a wood fire. Lead, in the form of Venetian ceruse, was extensively used in cosmetics by Western European aristocracy as whitened faces were regarded as a sign of modesty. This practice later expanded to white wigs and eyeliners, and only faded out with the French Revolution in the late 18th century. A similar fashion appeared in Japan in the 18th century with the emergence of the geishas, a practice that continued long into the 20th century. The white faces of women "came to represent their feminine virtue as Japanese women", with lead commonly used in the whitener.
Outside Europe and Asia
In the New World, lead production was recorded soon after the arrival of European settlers. The earliest record dates to 1621 in the English Colony of Virginia, fourteen years after its foundation. In Australia, the first mine opened by colonists on the continent was a lead mine, in 1841. In Africa, lead mining and smelting were known in the Benue Trough and the lower Congo Basin, where lead was used for trade with Europeans, and as a currency by the 17th century, well before the scramble for Africa.
Industrial Revolution

In the second half of the 18th century, Britain, and later continental Europe and the United States, experienced the
Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
. This was the first time during which lead production rates exceeded those of Rome. Britain was the leading producer, losing this status by the mid-19th century with the depletion of its mines and the development of lead mining in Germany, Spain, and the United States. By 1900, the United States was the leader in global lead production, and other non-European nations—Canada, Mexico, and Australia—had begun significant production; production outside Europe exceeded that within. A great share of the demand for lead came from plumbing and painting—
lead paint
Lead paint or lead-based paint is paint containing lead. As pigment, lead(II) chromate (, "chrome yellow"), lead(II,IV) oxide, (, "red lead"), and lead(II) carbonate (, "white lead") are the most common forms.. Lead is added to paint to acceler ...
s were in regular use. At this time, more (working class) people were exposed to the metal and lead poisoning cases escalated. This led to research into the effects of lead intake. Lead was proven to be more dangerous in its fume form than as a solid metal. Lead poisoning and gout were linked; British physician Alfred Baring Garrod noted a third of his gout patients were plumbers and painters. The effects of chronic ingestion of lead, including mental disorders, were also studied in the 19th century. The first laws aimed at decreasing lead poisoning in factories were enacted during the 1870s and 1880s in the United Kingdom.
Modern era

Further evidence of the threat that lead posed to humans was discovered in the late 19th and early 20th centuries. Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe. The United Kingdom introduced mandatory factory inspections in 1878 and appointed the first Medical Inspector of Factories in 1898; as a result, a 25-fold decrease in lead poisoning incidents from 1900 to 1944 was reported. Most European countries banned lead paint—commonly used because of its opacity and water resistance—for interiors by 1930.
The last major human exposure to lead was the addition of
tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
to gasoline as an antiknock agent, a practice that originated in the United States in 1921. It was phased out in the United States and the European Union by 2000.
In the 1970s, the United States and Western European countries introduced legislation to reduce lead air pollution. The impact was significant: while a study conducted by the Centers for Disease Control and Prevention in the United States in 1976–1980 showed that 77.8% of the population had elevated blood lead levels, in 1991–1994, a study by the same institute showed the share of people with such high levels dropped to 2.2%. The main product made of lead by the end of the 20th century was the Lead-acid battery, lead–acid battery.
From 1960 to 1990, lead output in the Western Bloc grew by about 31%. The share of the world's lead production by the Eastern Bloc increased from 10% to 30%, from 1950 to 1990, with the Soviet Union being the world's largest producer during the mid-1970s and the 1980s, and China starting major lead production in the late 20th century. Unlike the European communist countries, China was largely unindustrialized by the mid-20th century; in 2004, China surpassed Australia as the largest producer of lead. As was the case during European industrialization, lead has had a negative effect on health in China.
Production

As of 2014, production of lead is increasing worldwide due to its use in lead–acid batteries. There are two major categories of production: primary from mined ores, and secondary from scrap. In 2014, 4.58 million metric tons came from primary production and 5.64 million from secondary production. The top three producers of mined lead concentrate in that year were China, Australia, and United States. The top three producers of refined lead were China, United States, and India. According to the Metal Stocks in Society report of 2010, the total amount of lead in use, stockpiled, discarded, or dissipated into the environment, on a global basis, is 8 kg per capita. Much of this is in more developed countries (20–150 kg per capita) rather than less developed ones (1–4 kg per capita).
The primary and secondary lead production processes are similar. Some primary production plants now supplement their operations with scrap lead, and this trend is likely to increase in the future. Given adequate techniques, lead obtained via secondary processes is indistinguishable from lead obtained via primary processes. Scrap lead from the building trade is usually fairly clean and is re-melted without the need for smelting, though refining is sometimes needed. Secondary lead production is therefore cheaper, in terms of energy requirements, than is primary production, often by 50% or more.
Primary
Most lead ores contain a low percentage of lead (rich ores have a typical content of 3–8%) which must be concentrated for extraction. During initial processing, ores typically undergo crushing, dense-medium separation, grinding (abrasive cutting), grinding, froth flotation, drying. The resulting concentrate, which has a lead content of 30–80% by mass (regularly 50–60%), is then turned into (impure) lead metal.
There are two main ways of doing this: a two-stage process involving roasting followed by blast furnace extraction, carried out in separate vessels; or a direct process in which the extraction of the concentrate occurs in a single vessel. The latter has become the most common route, though the former is still significant.
calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
and magnesium. The resulting bismuth dross can be skimmed off.
Alternatively to the pyrometallurgical processes, very pure lead can be obtained by processing smelted lead electrolytically using the Betts electrolytic process, Betts process. Anodes of impure lead and cathodes of pure lead are placed in an electrolyte of lead Hexafluorosilicic acid, fluorosilicate (PbSiF
6). Once electrical potential is applied, impure lead at the anode dissolves and plates onto the cathode, leaving the majority of the impurities in solution. This is a high-cost process and thus mostly reserved for refining bullion containing high percentages of impurities.
Direct process
In this process, lead bullion and slag is obtained directly from lead concentrates. The lead sulfide concentrate is melted in a furnace and oxidized, forming lead monoxide. Carbon (as coke or coal gas) is added to the molten charge along with flux (metallurgy), fluxing agents. The lead monoxide is thereby reduced to metallic lead, in the midst of a slag rich in lead monoxide.
If the input is rich in lead, as much as 80% of the original lead can be obtained as bullion; the remaining 20% forms a slag rich in lead monoxide. For a low-grade feed, all of the lead can be oxidized to a high-lead slag. Metallic lead is further obtained from the high-lead (25–40%) slags via submerged fuel combustion or injection, reduction assisted by an electric furnace, or a combination of both.
Alternatives
Research on a cleaner, less energy-intensive lead extraction process continues; a major drawback is that either too much lead is lost as waste, or the alternatives result in a high sulfur content in the resulting lead metal. Hydrometallurgy, Hydrometallurgical extraction, in which anodes of impure lead are immersed into an electrolyte and pure lead is deposited (Electrowinning, electrowound) onto Cathode, cathodes, is a technique that may have potential, but is not currently economical except in cases where electricity is very cheap.
Secondary
Smelting, which is an essential part of the primary production, is often skipped during secondary production. It is only performed when metallic lead has undergone significant oxidation. The process is similar to that of primary production in either a blast furnace or a Rotary kiln, rotary furnace, with the essential difference being the greater variability of yields: blast furnaces produce hard lead (10% antimony) while reverberatory and rotary kiln furnaces produce semisoft lead (3–4% antimony).
The ISASMELT process is a more recent smelting method that may act as an extension to primary production; battery paste from spent lead–acid batteries (containing lead sulfate and lead oxides) has its sulfate removed by treating it with alkali, and is then treated in a coal-fueled furnace in the presence of oxygen, which yields impure lead, with antimony the most common impurity. Refining of secondary lead is similar to that of primary lead; some refining processes may be skipped depending on the material recycled and its potential contamination.
Of the sources of lead for recycling, lead–acid batteries are the most important; lead pipe, sheet, and cable sheathing are also significant.
Applications

Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. When the pencil originated as a wrapped graphite writing tool, the particular type of graphite used was named ''Graphite, plumbago'' (literally, ''lead mockup'').
Elemental form
Lead metal has several useful mechanical properties, including high density, low melting point, ductility, and relative inertness. Many metals are superior to lead in some of these aspects but are generally less common and more difficult to extract from parent ores. Lead's toxicity has led to its phasing out for some uses.
Lead has been used for bullets since their invention in the Middle Ages. It is inexpensive; its low melting point means small arms ammunition and shotgun pellets can be cast with minimal technical equipment; and it is denser than other common metals, which allows for better retention of velocity. It remains the main material for bullets, alloyed with other metals as hardeners. Concerns have been raised that lead bullets used for hunting can damage the environment. Shotgun Shotgun cartridge, cartridges used for waterfowl hunting must today be lead-free in the United States, Canada, and in Europe.
Lead's high density and resistance to corrosion have been exploited in a number of related applications. It is used as ballast in sailboat keels; its density allows it to take up a small volume and minimize water resistance, thus counterbalancing the heeling effect of wind on the sails. It is used in scuba diving diving weighting system, weight belts to counteract the diver's buoyancy. In 1993, the base of the Leaning Tower of Pisa was stabilized with 600 tonnes of lead. Because of its corrosion resistance, lead is used as a protective sheath for underwater cables.
Lead has many uses in the construction industry; lead sheets are used as architectural metals in Domestic roof construction, roofing material, Cladding (construction), cladding, Flashing (weatherproofing), flashing, rain gutter, gutters and gutter joints, roof parapets. Lead is still used in statues and sculptures, including for armature (sculpture), armatures. In the past it was often used to tire balance, balance the wheels of cars; for environmental reasons this use is being phased out in favor of other materials.
Lead is added to copper alloys, such as brass and bronze, to improve machinability and for its lubricating qualities. Being practically insoluble in copper, the lead forms solid globules in imperfections throughout the alloy, such as Grain boundary, grain boundaries. In low concentrations, as well as acting as a lubricant, the globules hinder the formation of swarf as the alloy is worked, thereby improving machinability. Copper alloys with larger concentrations of lead are used in Bearing (mechanical), bearings. The lead provides lubrication, and the copper provides the load-bearing support.
Lead's high density, atomic number, and formability form the basis for use of lead as a barrier that absorbs sound, vibration, and radiation. Lead has no natural resonance frequencies; as a result, sheet-lead is used as a sound deadening layer in the walls, floors, and ceilings of sound studios. Organ pipes are often made from a lead alloy, mixed with various amounts of tin to control the tone of each pipe. Lead is an established lead shielding, shielding material from ionizing radiation, radiation in nuclear science and in X-ray rooms due to its denseness and high attenuation coefficient. Molten lead has been used as a coolant for lead-cooled fast reactors.
Batteries
The largest use of lead in the early 21st century is in Lead-acid battery, lead–acid batteries. The lead in batteries undergoes no direct contact with humans, so there are fewer toxicity concerns. People who work in lead battery production or recycling plants may be exposed to lead dust and inhale it. The reactions in the battery between lead, lead dioxide, and sulfuric acid provide a reliable source of voltage. Supercapacitors incorporating lead–acid batteries have been installed in kilowatt and megawatt scale applications in Australia, Japan, and the United States in frequency regulation, solar smoothing and shifting, wind smoothing, and other applications. These batteries have lower energy density and charge-discharge efficiency than lithium-ion battery, lithium-ion batteries, but are significantly cheaper.
Coating for cables
Lead is used in high voltage power cables as shell material to prevent water diffusion into insulation; this use is decreasing as lead is being phased out. Its use in
solder
Solder (; North American English, NA: ) is a fusible alloy, fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces aft ...
for electronics is also being phased out by some countries to reduce the amount of environmental hazard, environmentally hazardous waste. Lead is one of three metals used in the Oddy test for museum materials, helping detect organic acids, aldehydes, acidic gases.
Compounds

In addition to being the main application for lead metal, lead–acid batteries are also the main consumer of lead compounds. The energy storage/release reaction used in these devices involves Lead(II) sulfate, lead sulfate and lead dioxide:
:(s) + (s) + 2(aq) → 2(s) + 2(l)
Other applications of lead compounds are very specialized and often fading. Lead-based coloring agents are used in ceramic glazes and glass, especially for red and yellow shades. While lead paints are phased out in Europe and North America, they remain in use in less developed countries such as China, India, or Indonesia. Lead tetraacetate and lead dioxide are used as oxidizing agents in organic chemistry. Lead is frequently used in the polyvinyl chloride coating of electrical cords. It can be used to treat candle wicks to ensure a longer, more even burn. Because of its toxicity, European and North American manufacturers use alternatives such as zinc. Lead glass is composed of 12–28% Lead(II) oxide, lead oxide, changing its optical characteristics and reducing the transmission of ionizing radiation, a property used in old TVs and computer monitors with cathode-ray tubes. Lead-based
semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
s such as
lead telluride and
lead selenide are used in Photovoltaics, photovoltaic cells and infrared detectors.
Biological effects
Lead has no confirmed biological role, and there is no confirmed safe level of lead exposure. A 2009 Canadian–American study concluded that even at levels that are considered to pose little to no risk, lead may cause "adverse mental health outcomes". Its prevalence in the human body—at an adult average of 120 mg—is nevertheless exceeded only by zinc (2500 mg) and iron (4000 mg) among the heavy metals. Lead Salt (chemistry), salts are very efficiently absorbed by the body. A small amount of lead (1%) is stored in bones; the rest is excreted in urine and feces within a few weeks of exposure. Only about a third of lead is excreted by a child. Continual exposure may result in the bioaccumulation of lead.
Toxicity
Lead is a highly poisonous metal (whether inhaled or swallowed), affecting almost every organ and system in the human body. At airborne levels of 100 mg/m
3, it is Immediately dangerous to life or health, immediately dangerous to life and health. Most ingested lead is absorbed into the bloodstream. The primary cause of its toxicity is its predilection for interfering with the proper functioning of enzymes. It does so by binding to the Thiol, sulfhydryl groups found on many enzymes, or mimicking and displacing other metals which act as cofactor (biochemistry), cofactors in many enzymatic reactions. The essential metals that lead interacts with include calcium, iron, and zinc. High levels of calcium and iron tend to provide some protection from lead poisoning; low levels cause increased susceptibility.
Effects
Lead can cause severe damage to the brain and kidneys and, ultimately, death. By mimicking calcium, lead can cross the blood–brain barrier. It degrades the myelin sheaths of neurons, reduces their numbers, interferes with neurotransmitter, neurotransmission routes, and decreases neuronal growth. In the human body, lead inhibits Delta-aminolevulinic acid dehydratase, porphobilinogen synthase and ferrochelatase, preventing both porphobilinogen formation and the incorporation of iron into protoporphyrin IX, the final step in heme synthesis. This causes ineffective heme synthesis and microcytic anemia.
Symptoms of lead poisoning include Kidney disease, nephropathy, colic-like abdominal pains, and possibly weakness in the fingers, wrists, or ankles. Small blood pressure increases, particularly in middle-aged and older people, may be apparent and can cause anemia. Several studies, mostly cross-sectional, found an association between increased lead exposure and decreased heart rate variability. In pregnant women, high levels of exposure to lead may cause miscarriage. Chronic, high-level exposure has been shown to reduce fertility in males.
In a child's developing brain, lead interferes with synapse formation in the cerebral cortex, neurochemical development (including that of neurotransmitters), and the organization of ion channels. Early childhood exposure has been linked with an increased risk of sleep disturbances and excessive daytime drowsiness in later childhood. High blood levels are associated with delayed puberty in girls. The rise and fall in exposure to airborne lead from the combustion of tetraethyl lead in gasoline during the 20th century has been linked with historical increases and Lead–crime hypothesis, decreases in crime levels.
Exposure sources
Lead exposure is a global issue since lead mining and smelting, and battery manufacturing, disposal, and Battery recycling#Lead.E2.80.93acid batteries, recycling, are common in many countries. Lead enters the body via inhalation, ingestion, or skin absorption. Almost all inhaled lead is absorbed into the body; for ingestion, the rate is 20–70%, with children absorbing a higher percentage than adults.
Poisoning typically results from ingestion of food or water contaminated with lead, and less commonly after accidental ingestion of contaminated soil, dust, or lead-based paint. Seawater products can contain lead if affected by nearby industrial waters. Fruit and vegetables can be contaminated by high levels of lead in the soils they were grown in. Soil can be contaminated through particulate accumulation from lead in pipes,
lead paint
Lead paint or lead-based paint is paint containing lead. As pigment, lead(II) chromate (, "chrome yellow"), lead(II,IV) oxide, (, "red lead"), and lead(II) carbonate (, "white lead") are the most common forms.. Lead is added to paint to acceler ...
, residual emissions from leaded gasoline.
The use of lead for water pipes is plumbosolvency, a problem in areas with soft or acidic water. Hard water forms insoluble protective layers on the inner surface of the pipes, whereas soft and acidic water dissolves the lead pipes. Dissolved carbon dioxide in the carried water may result in the formation of soluble lead bicarbonate; oxygenated water may similarly dissolve lead as lead(II) hydroxide. Drinking such water, over time, can cause health problems due to the toxicity of the dissolved lead. The hard water, harder the water the more calcium bicarbonate and calcium sulfate, sulfate it contains, and the more the inside of the pipes are coated with a protective layer of lead carbonate or lead sulfate.

Ingestion of applied lead-based paint is the major source of exposure for children: a direct source is chewing on old painted window sills. Additionally, as lead paint on a surface deteriorates, it peels and is pulverized into dust. The dust then enters the body through hand-to-mouth contact or contaminated food or drink. Ingesting certain Traditional medicine#Home remedies, home remedies may result in exposure to lead or its compounds.
Inhalation is the second major exposure pathway, affecting smokers and especially workers in lead-related occupations. Tobacco smoke, Cigarette smoke contains, among other toxic substances, radioactive Isotopes of lead, lead-210. "As a result of EPA's regulatory efforts, levels of lead in the air [in the United States] decreased by 86 percent between 2010 and 2020."
The concentration of lead in the air in the United States fell below the national standard of 0.15 μg/m
3 in 2014.
Skin exposure may be significant for people working with organic lead compounds. The rate of skin absorption is lower for inorganic lead.
Lead in foods
Lead may be found in food when food is grown in soil that is high in lead, airborne lead contaminates the crops, animals eat lead in their diet, or lead enters the food either from what it was stored or cooked in. Ingestion of lead paint and batteries is also a route of exposure for livestock, which can subsequently affect humans. Milk produced by contaminated cattle can be diluted to a lower lead concentration and sold for consumption.
In Bangladesh, lead compounds have been added to turmeric to make it more yellow.
This is believed to have started in the 1980s and continues .
[ It is believed to be one of the main sources of high lead levels in the country. In Hong Kong the maximum allowed lead level in food is 6 parts per million in solids and 1 part per million in liquids.
Lead-containing dust can settle on drying cocoa beans when they are set outside near polluting industrial plants. In December 2022, Consumer Reports tested 28 dark chocolate brands and found that 23 of them contained potentially harmful levels of lead, cadmium or both. They have urged the chocolate makers to reduce the level of lead which could be harmful, especially to a developing fetus.
In March 2024, the US Food and Drug Administration recommended a voluntary recall on 6 brands of cinnamon due to contamination with lead, after 500 reports of child lead poisoning. The FDA determined that cinnamon was adulterated with lead chromate.
]
Lead in plastic toys
According to the United States Centers for Disease Control and Prevention, Center for Disease Control, the use of lead in plastics has not been banned as of 2024. Lead softens the plastic and makes it more flexible so that it can go back to its original shape. Habitual chewing on colored plastic insulation from stripped electrical wires was found to cause elevated lead levels in a 46-year-old man. Lead may be used in plastic toys to stabilize molecules from heat. Lead dust can be formed when plastic is exposed to sunlight, air, and detergents that break down the chemical bond between the lead and plastics.
Treatment
Treatment for lead poisoning normally involves the administration of dimercaprol and succimer. Acute cases may require the use of Sodium calcium edetate, disodium calcium edetate, the calcium Chelation, chelate, and the disodium salt of ethylenediaminetetraacetic acid (Ethylenediaminetetraacetic acid, EDTA). It has a greater affinity for lead than calcium, with the result that lead chelate is formed by exchange and excreted in the urine, leaving behind harmless calcium.
Environmental effects
 The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting photosynthesis and at high enough concentrations, negatively affecting plant growth and survival. Contamination of soils and plants can allow lead to ascend the food chain affecting microorganisms and animals. In animals, lead exhibits toxicity in many organs, damaging the nervous, kidney, renal, reproductive, Haematopoiesis, hematopoietic, and cardiovascular systems after ingestion, inhalation, or skin absorption. Fish uptake lead from both water and sediment; bioaccumulation in the food chain poses a hazard to fish, birds, and sea mammals.
Anthropogenic lead includes lead from Shot (pellet), shot and Fishing sinker, sinkers. These are among the most potent sources of lead contamination along with lead production sites. Lead was banned for shot and sinkers in the United States in 2017, although that ban was only effective for a month, and a similar ban is being considered in the European Union.
Analytical methods for the determination of lead in the environment include spectrophotometry, X-ray fluorescence, atomic spectroscopy, and electrochemistry, electrochemical methods. A specific ion-selective electrode has been developed based on the ionophore ''S'',''S-methylenebis(''N'',''N''-diisobutyldithiocarbamate). An important biomarker assay for lead poisoning is δ-aminolevulinic acid levels in plasma, serum, and urine.
The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting photosynthesis and at high enough concentrations, negatively affecting plant growth and survival. Contamination of soils and plants can allow lead to ascend the food chain affecting microorganisms and animals. In animals, lead exhibits toxicity in many organs, damaging the nervous, kidney, renal, reproductive, Haematopoiesis, hematopoietic, and cardiovascular systems after ingestion, inhalation, or skin absorption. Fish uptake lead from both water and sediment; bioaccumulation in the food chain poses a hazard to fish, birds, and sea mammals.
Anthropogenic lead includes lead from Shot (pellet), shot and Fishing sinker, sinkers. These are among the most potent sources of lead contamination along with lead production sites. Lead was banned for shot and sinkers in the United States in 2017, although that ban was only effective for a month, and a similar ban is being considered in the European Union.
Analytical methods for the determination of lead in the environment include spectrophotometry, X-ray fluorescence, atomic spectroscopy, and electrochemistry, electrochemical methods. A specific ion-selective electrode has been developed based on the ionophore ''S'',''S-methylenebis(''N'',''N''-diisobutyldithiocarbamate). An important biomarker assay for lead poisoning is δ-aminolevulinic acid levels in plasma, serum, and urine.
Restriction and remediation
By the mid-1980s, there was significant decline in the use of lead in industry. In the United States, environmental regulations reduced or eliminated the use of lead in non-battery products, including gasoline, paints, solders, and water systems. Scrubber, Particulate control devices were installed in Coal-fired power station, coal-fired power plants to capture lead emissions. In 1992, U.S. Congress required the Environmental Protection Agency to reduce the blood lead levels of the country's children. Lead use was further curtailed by the European Union's 2003 Restriction of Hazardous Substances Directive. A large drop in lead deposition occurred in the Netherlands after the 1993 national ban on use of lead shot for hunting and sport shooting: from 230 tonnes in 1990 to 47.5 tonnes in 1995. The usage of lead in Avgas#100LL (blue), Avgas 100LL for general aviation is allowed in the EU as of 2022.zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
in its shoot and root tissues making it an important plant for remediation of contaminated soils.
See also
* Derek Bryce-Smith, Derek Bryce-Smith – one of the earliest campaigners against lead in petrol in the UK
* Thomas Midgley Jr. – discovered that the addition of tetraethyllead
Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...
to gasoline prevented Engine knocking, "knocking" in internal combustion engines
* Clair Patterson, Clair Cameron Patterson – instrumental in the banning of tetraethyllead in gasoline in the US and lead solder in food cans.
* Robert A. Kehoe – foremost medical advocate for the use of tetraethyllead as an additive in gasoline.
Notes
References
Bibliography
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
Further reading
*
Table of contents
*
*
* Ingalls, Walter Renton (1865-)
Lead Smelting and Refining, With Some Notes on Lead Mining
*
External links
The Toxicology of Heavy Metals: Getting the Lead Out
American Society for Clinical Pathology
COVID-19 to reduce global lead production by 5,2% in 2020 (with a figure showing global lead production, 2010–2024)
{{Authority control
Lead,
Chemical elements
Hazardous materials
Post-transition metals
Native element minerals
Superconductors
Endocrine disruptors
IARC Group 2B carcinogens
Nuclear reactor coolants
Chemical elements with face-centered cubic structure
 The melting point of lead—at 327.5 °C (621.5 °F)—is very low compared to most metals. Its
The melting point of lead—at 327.5 °C (621.5 °F)—is very low compared to most metals. Its  Three of the stable isotopes are found in three of the four major
Three of the stable isotopes are found in three of the four major  Lead dihalides are well-characterized; this includes the diastatide and mixed halides, such as PbFCl. The relative insolubility of the latter forms a useful basis for the gravimetric determination of fluorine. The difluoride was the first solid ionically conducting compound to be discovered (in 1834, by
Lead dihalides are well-characterized; this includes the diastatide and mixed halides, such as PbFCl. The relative insolubility of the latter forms a useful basis for the gravimetric determination of fluorine. The difluoride was the first solid ionically conducting compound to be discovered (in 1834, by  Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained, as an intermediate between lead(II) and lead(IV), in larger organolead complexes; this oxidation state is not stable, as both the lead(III) ion and the larger complexes containing it are radicals. The same applies for lead(I), which can be found in such radical species.
Numerous mixed lead(II,IV) oxides are known. When PbO2 is heated in air, it becomes Pb12O19 at 293 °C, Pb12O17 at 351 °C, Pb3O4 at 374 °C, and finally PbO at 605 °C. A further sesquioxide, Pb2O3, can be obtained at high pressure, along with several non-stoichiometric phases. Many of them show defective
Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained, as an intermediate between lead(II) and lead(IV), in larger organolead complexes; this oxidation state is not stable, as both the lead(III) ion and the larger complexes containing it are radicals. The same applies for lead(I), which can be found in such radical species.
Numerous mixed lead(II,IV) oxides are known. When PbO2 is heated in air, it becomes Pb12O19 at 293 °C, Pb12O17 at 351 °C, Pb3O4 at 374 °C, and finally PbO at 605 °C. A further sesquioxide, Pb2O3, can be obtained at high pressure, along with several non-stoichiometric phases. Many of them show defective  Lead can form multiply-bonded chains, a property it shares with its lighter homologs in the carbon group. Its capacity to do so is much less because the Pb–Pb
Lead can form multiply-bonded chains, a property it shares with its lighter homologs in the carbon group. Its capacity to do so is much less because the Pb–Pb  Roman Republic, Rome's territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the Classical antiquity, classical era, with an estimated annual output peaking at 80,000 tonnes. Like their predecessors, the Romans obtained lead mostly as a by-product of silver smelting. Lead mining occurred in central Europe, Roman Britain, Britain, Balkans, Greece, Anatolia, Hispania, the latter accounting for 40% of world production.
Roman Republic, Rome's territorial expansion in Europe and across the Mediterranean, and its development of mining, led to it becoming the greatest producer of lead during the Classical antiquity, classical era, with an estimated annual output peaking at 80,000 tonnes. Like their predecessors, the Romans obtained lead mostly as a by-product of silver smelting. Lead mining occurred in central Europe, Roman Britain, Britain, Balkans, Greece, Anatolia, Hispania, the latter accounting for 40% of world production.
 Lead tablets were commonly used as a material for letters. Lead coffins, cast in flat sand forms and with interchangeable motifs to suit the faith of the deceased, were used in ancient Judaea (Roman province), Judea. Lead was used to make sling bullets from the 5th century BC. In Roman times, lead sling bullets were amply used, and were effective at a distance of between 100 and 150 meters. The Balearic slinger, Balearic slingers, used as mercenaries in Carthaginian and Roman armies, were famous for their shooting distance and accuracy.
Lead was used for making water pipes in the Roman Empire; the
Lead tablets were commonly used as a material for letters. Lead coffins, cast in flat sand forms and with interchangeable motifs to suit the faith of the deceased, were used in ancient Judaea (Roman province), Judea. Lead was used to make sling bullets from the 5th century BC. In Roman times, lead sling bullets were amply used, and were effective at a distance of between 100 and 150 meters. The Balearic slinger, Balearic slingers, used as mercenaries in Carthaginian and Roman armies, were famous for their shooting distance and accuracy.
Lead was used for making water pipes in the Roman Empire; the  In the second half of the 18th century, Britain, and later continental Europe and the United States, experienced the
In the second half of the 18th century, Britain, and later continental Europe and the United States, experienced the  Further evidence of the threat that lead posed to humans was discovered in the late 19th and early 20th centuries. Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe. The United Kingdom introduced mandatory factory inspections in 1878 and appointed the first Medical Inspector of Factories in 1898; as a result, a 25-fold decrease in lead poisoning incidents from 1900 to 1944 was reported. Most European countries banned lead paint—commonly used because of its opacity and water resistance—for interiors by 1930.
The last major human exposure to lead was the addition of
Further evidence of the threat that lead posed to humans was discovered in the late 19th and early 20th centuries. Mechanisms of harm were better understood, lead blindness was documented, and the element was phased out of public use in the United States and Europe. The United Kingdom introduced mandatory factory inspections in 1878 and appointed the first Medical Inspector of Factories in 1898; as a result, a 25-fold decrease in lead poisoning incidents from 1900 to 1944 was reported. Most European countries banned lead paint—commonly used because of its opacity and water resistance—for interiors by 1930.
The last major human exposure to lead was the addition of  Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. When the pencil originated as a wrapped graphite writing tool, the particular type of graphite used was named ''Graphite, plumbago'' (literally, ''lead mockup'').
Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. When the pencil originated as a wrapped graphite writing tool, the particular type of graphite used was named ''Graphite, plumbago'' (literally, ''lead mockup'').
 In addition to being the main application for lead metal, lead–acid batteries are also the main consumer of lead compounds. The energy storage/release reaction used in these devices involves Lead(II) sulfate, lead sulfate and lead dioxide:
:(s) + (s) + 2(aq) → 2(s) + 2(l)
Other applications of lead compounds are very specialized and often fading. Lead-based coloring agents are used in ceramic glazes and glass, especially for red and yellow shades. While lead paints are phased out in Europe and North America, they remain in use in less developed countries such as China, India, or Indonesia. Lead tetraacetate and lead dioxide are used as oxidizing agents in organic chemistry. Lead is frequently used in the polyvinyl chloride coating of electrical cords. It can be used to treat candle wicks to ensure a longer, more even burn. Because of its toxicity, European and North American manufacturers use alternatives such as zinc. Lead glass is composed of 12–28% Lead(II) oxide, lead oxide, changing its optical characteristics and reducing the transmission of ionizing radiation, a property used in old TVs and computer monitors with cathode-ray tubes. Lead-based
In addition to being the main application for lead metal, lead–acid batteries are also the main consumer of lead compounds. The energy storage/release reaction used in these devices involves Lead(II) sulfate, lead sulfate and lead dioxide:
:(s) + (s) + 2(aq) → 2(s) + 2(l)
Other applications of lead compounds are very specialized and often fading. Lead-based coloring agents are used in ceramic glazes and glass, especially for red and yellow shades. While lead paints are phased out in Europe and North America, they remain in use in less developed countries such as China, India, or Indonesia. Lead tetraacetate and lead dioxide are used as oxidizing agents in organic chemistry. Lead is frequently used in the polyvinyl chloride coating of electrical cords. It can be used to treat candle wicks to ensure a longer, more even burn. Because of its toxicity, European and North American manufacturers use alternatives such as zinc. Lead glass is composed of 12–28% Lead(II) oxide, lead oxide, changing its optical characteristics and reducing the transmission of ionizing radiation, a property used in old TVs and computer monitors with cathode-ray tubes. Lead-based  Ingestion of applied lead-based paint is the major source of exposure for children: a direct source is chewing on old painted window sills. Additionally, as lead paint on a surface deteriorates, it peels and is pulverized into dust. The dust then enters the body through hand-to-mouth contact or contaminated food or drink. Ingesting certain Traditional medicine#Home remedies, home remedies may result in exposure to lead or its compounds.
Inhalation is the second major exposure pathway, affecting smokers and especially workers in lead-related occupations. Tobacco smoke, Cigarette smoke contains, among other toxic substances, radioactive Isotopes of lead, lead-210. "As a result of EPA's regulatory efforts, levels of lead in the air [in the United States] decreased by 86 percent between 2010 and 2020." The concentration of lead in the air in the United States fell below the national standard of 0.15 μg/m3 in 2014.
Skin exposure may be significant for people working with organic lead compounds. The rate of skin absorption is lower for inorganic lead.
Ingestion of applied lead-based paint is the major source of exposure for children: a direct source is chewing on old painted window sills. Additionally, as lead paint on a surface deteriorates, it peels and is pulverized into dust. The dust then enters the body through hand-to-mouth contact or contaminated food or drink. Ingesting certain Traditional medicine#Home remedies, home remedies may result in exposure to lead or its compounds.
Inhalation is the second major exposure pathway, affecting smokers and especially workers in lead-related occupations. Tobacco smoke, Cigarette smoke contains, among other toxic substances, radioactive Isotopes of lead, lead-210. "As a result of EPA's regulatory efforts, levels of lead in the air [in the United States] decreased by 86 percent between 2010 and 2020." The concentration of lead in the air in the United States fell below the national standard of 0.15 μg/m3 in 2014.
Skin exposure may be significant for people working with organic lead compounds. The rate of skin absorption is lower for inorganic lead.
 The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting photosynthesis and at high enough concentrations, negatively affecting plant growth and survival. Contamination of soils and plants can allow lead to ascend the food chain affecting microorganisms and animals. In animals, lead exhibits toxicity in many organs, damaging the nervous, kidney, renal, reproductive, Haematopoiesis, hematopoietic, and cardiovascular systems after ingestion, inhalation, or skin absorption. Fish uptake lead from both water and sediment; bioaccumulation in the food chain poses a hazard to fish, birds, and sea mammals.
Anthropogenic lead includes lead from Shot (pellet), shot and Fishing sinker, sinkers. These are among the most potent sources of lead contamination along with lead production sites. Lead was banned for shot and sinkers in the United States in 2017, although that ban was only effective for a month, and a similar ban is being considered in the European Union.
Analytical methods for the determination of lead in the environment include spectrophotometry, X-ray fluorescence, atomic spectroscopy, and electrochemistry, electrochemical methods. A specific ion-selective electrode has been developed based on the ionophore ''S'',''S-methylenebis(''N'',''N''-diisobutyldithiocarbamate). An important biomarker assay for lead poisoning is δ-aminolevulinic acid levels in plasma, serum, and urine.
The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters. Atmospheric emissions of lead were at their peak during the Industrial Revolution, and the leaded gasoline period in the second half of the twentieth century.
Lead releases originate from natural sources (i.e., concentration of the naturally occurring lead), industrial production, incineration and recycling, and mobilization of previously buried lead. In particular, as lead has been phased out from other uses, in the Global South, lead recycling operations designed to extract cheap lead used for global manufacturing have become a well documented source of exposure. Elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas; industrial emissions, including those arising from coal burning, continue in many parts of the world, particularly in the developing countries.
Lead can accumulate in soils, especially those with a high organic content, where it remains for hundreds to thousands of years. Environmental lead can compete with other metals found in and on plant surfaces potentially inhibiting photosynthesis and at high enough concentrations, negatively affecting plant growth and survival. Contamination of soils and plants can allow lead to ascend the food chain affecting microorganisms and animals. In animals, lead exhibits toxicity in many organs, damaging the nervous, kidney, renal, reproductive, Haematopoiesis, hematopoietic, and cardiovascular systems after ingestion, inhalation, or skin absorption. Fish uptake lead from both water and sediment; bioaccumulation in the food chain poses a hazard to fish, birds, and sea mammals.
Anthropogenic lead includes lead from Shot (pellet), shot and Fishing sinker, sinkers. These are among the most potent sources of lead contamination along with lead production sites. Lead was banned for shot and sinkers in the United States in 2017, although that ban was only effective for a month, and a similar ban is being considered in the European Union.
Analytical methods for the determination of lead in the environment include spectrophotometry, X-ray fluorescence, atomic spectroscopy, and electrochemistry, electrochemical methods. A specific ion-selective electrode has been developed based on the ionophore ''S'',''S-methylenebis(''N'',''N''-diisobutyldithiocarbamate). An important biomarker assay for lead poisoning is δ-aminolevulinic acid levels in plasma, serum, and urine.