|
Zinc–carbon Battery
A zinc–carbon battery (or carbon zinc battery in U.S. English) is the generic “heavy duty” disposable battery. It has been overtaken in recent times by the longer-lasting alkaline battery. A zinc–carbon battery is a dry cell that provides direct electric current from the electrochemical reaction between zinc (Zn) and manganese dioxide (MnO2) in the presence of an ammonium chloride (NH4Cl) electrolyte. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by a compound with a higher Standard electrode potential (positive polarity), known as the cathode, that collects the current from the manganese dioxide electrode. The name "zinc–carbon" is slightly misleading as it implies that carbon is acting as the oxidizing agent rather than the manganese dioxide. General-purpose batteries may use an acidic aqueous paste of ammonium chloride (NH4Cl) as electro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional current describes the direction in which positive charges move. Electrons, which are the carriers of current in most electrical systems, have a negative electrical charge, so the movement of electrons is ''opposite'' to that of the conventional current flow: this means that electrons flow ''into'' the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode. The electrode through which conventional current flows the other way, into the device, is termed an anode. Charge flow Conventional current flows from cathode to anode outside the cell or device (with electrons moving in the opposite direction), regardless of the cell or device type and operating mode. Cathode polarity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plaster Of Paris
Plaster is a building material used for the protective or decorative coating of walls and ceilings and for moulding and casting decorative elements. In English, "plaster" usually means a material used for the interiors of buildings, while "render" commonly refers to external applications. The term stucco refers to plasterwork that is worked in some way to produce relief decoration, rather than flat surfaces. The most common types of plaster mainly contain either gypsum, lime, or cement,Franz Wirsching "Calcium Sulfate" in Ullmann's Encyclopedia of Industrial Chemistry, 2012 Wiley-VCH, Weinheim. but all work in a similar way. The plaster is manufactured as a dry powder and is mixed with water to form a stiff but workable paste immediately before it is applied to the surface. The reaction with water liberates heat through crystallization and the hydrated plaster then hardens. Plaster can be relatively easily worked with metal tools and sandpaper and can be moulded, either ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Gassner
Carl Gassner was a German physician (17 November 1855 in Mainz31 January 1942), scientist and inventor, better known to have contributed to improve the Leclanché cell and to have fostered the development of the first dry cell, also known as the zinc–carbon battery, less likely to break or leak and that could be effectively industrially produced at large scale. Life Gassner studied the medicine at the University of Strasbourg and then practiced in Mainz (Germany) as a specialist in diseases of the eyes and ears. He also conducted experiments in physics and chemistry at the Balbach watchmaking industry. Invention of dry cell In 1880, most of the door bells operated with a wet Leclanché cell containing an aqueous electrolyte solution which often dried out, rendering the cell unusable. To remediate this inconvenience, in 1876, Georges Leclanché started to jellify the electrolyte of his cell by adding starch to the ammonium chloride, making also his cell more portable. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
W Pil 001 Pile Wonder 3V Années 60
W, or w, is the twenty-third letter of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''double-u'',Pronounced in formal situations, but colloquially often , , or , with a silent ''l''. plural ''double-ues''. Name Double-u, whose name reflects stages in the letter's evolution when it was considered two of the same letter, a double U, is the only modern English letter whose name has more than one syllable.However, "Izzard" was formerly a two-syllable pronunciation of the letter Z. It is also the only English letter whose name is not pronounced with any of the sounds that the letter typically makes in words, with the exception of H (though not for all speakers, particularly in British English). Some speakers shorten the name "double u" into "dub-u" or just "dub"; for example, University of Wisconsin, University of Washington, University of Wyoming, University o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Cell
A primary battery or primary cell is a battery (a galvanic cell) that is designed to be used once and discarded, and it is not rechargeable unlike a secondary cell ( rechargeable battery). In general, the electrochemical reaction occurring in the cell is not reversible, rendering the cell unrechargeable. As a primary cell is used, chemical reactions in the battery use up the chemicals that generate the power; when they are gone, the battery stops producing electricity. In contrast, in a secondary cell, the reaction can be reversed by running a current into the cell with a battery charger to recharge it, regenerating the chemical reactants. Primary cells are made in a range of standard sizes to power small household appliances such as flashlights and portable radios. Primary batteries make up about 90% of the $50 billion battery market, but secondary batteries have been gaining market share. About 15 billion primary batteries are thrown away worldwide every year, virtually al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transistor Radio
A transistor radio is a small portable radio receiver that uses transistor-based circuitry. Previous portable radios used vacuum tubes, which were bulky, fragile, had a limited lifetime, consumed excessive power and required large heavy batteries. Following the History of the transistor, invention of the transistor in 1947—a semiconductor device that amplifies and acts as an electronic switch, which revolutionized the field of consumer electronics by introducing small but powerful, convenient hand-held devices—the Regency TR-1 was released in 1954 becoming the first commercial transistor radio. The mass-market success of the smaller and cheaper Sony TR-63, released in 1957, led to the transistor radio becoming the most popular electronic communication device of the 1960s and 1970s. Billions had been manufactured by about 2012. The pocket size of transistor radios sparked a change in popular music listening habits, allowing people to listen to music and other broadcasts o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Remote Control
A remote control, also known colloquially as a remote or clicker, is an consumer electronics, electronic device used to operate another device from a distance, usually wirelessly. In consumer electronics, a remote control can be used to operate devices such as a television set, DVD player or other digital home media appliance. A remote control can allow operation of devices that are out of convenient reach for direct operation of controls. They function best when used from a short distance. This is primarily a convenience feature for the user. In some cases, remote controls allow a person to operate a device that they otherwise would not be able to reach, as when a garage door opener is triggered from outside. Early television remote controls (1956–1977) used ultrasonics, ultrasonic tones. Present-day remote controls are commonly consumer IR, consumer infrared devices which send digitally-coded pulses of infrared radiation. They control functions such as power, volume, chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flashlight
A flashlight (US English) or electric torch (Commonwealth English), usually shortened to torch, is a portable hand-held electric lamp. Formerly, the light source typically was a miniature incandescent light bulb, but these have been displaced by light-emitting diodes (LEDs) since the early 2000s. A typical flashlight consists of the light source mounted in a reflector, a transparent cover (sometimes combined with a lens) to protect the light source and reflector, a battery, and a switch, all enclosed in a case. The invention of the dry cell and miniature incandescent electric lamps made the first battery-powered flashlights possible around 1899. Today, flashlights use mostly light-emitting diodes and run on disposable or rechargeable batteries. Some are powered by the user turning a crank, shaking the lamp, or squeezing it. Some have solar panels to recharge the battery. Flashlights are used as a light source outdoors, in places without permanently installed lighting, during ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
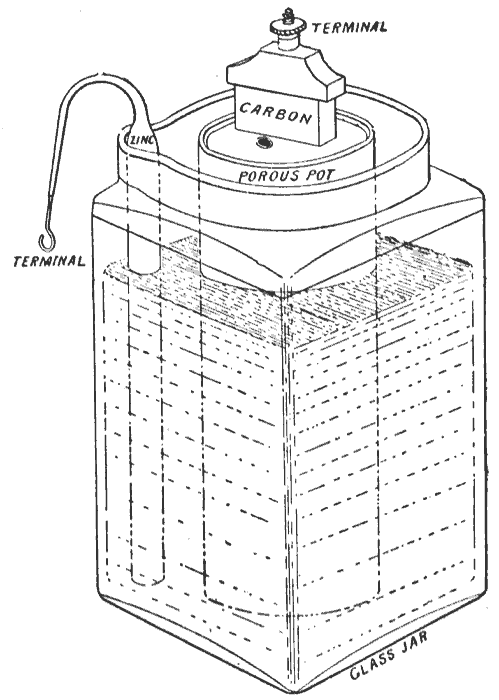
Leclanché Cell
The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode (positive terminal) of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode (negative terminal) of zinc (reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell. History In 1866, Georges Leclanché invented a battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts. This cell achieved very quick success in telegraphy, signalling and electric bell work. The dry cell form was used to power early telephones—usually from an adjacent wooden box affixed to the wall—before telephones could dr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Cell
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons. When a battery is connected to an external electric load, those negatively charged electrons flow through the circuit and reach the positive terminal, thus causing a redox reaction by attracting positively charged ions, or cations. Thus, higher energy reactants are converted to lower energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell. Primary (single-use or "disposable") batteries are used once and discarded, as the electrode m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |