Leclanché cell on:
[Wikipedia]
[Google]
[Amazon]
 The Leclanché cell is a battery invented and patented by the French scientist
The Leclanché cell is a battery invented and patented by the French scientist
Alternately, the reduction reaction of Mn(IV) can proceed further, forming Mn(II) hydroxide. :Zn(s) + MnO2(s) + 2 NH4Cl(aq) → ZnCl2(aq) + Mn(OH)2(s) + 2 NH3(aq)
 The Leclanché cell is a battery invented and patented by the French scientist
The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché
Georges Leclanché (9 October 1839 – 14 September 1882) was a French electrical engineer chiefly remembered for his invention of the Leclanché cell, one of the first modern electrical batteries and the forerunner of the modern dry cell battery ...
in 1866. The battery contained a conducting solution (electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
) of ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
, a cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
(positive terminal) of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, a depolarizer of manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cel ...
(oxidizer), and an anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
(negative terminal) of zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
(reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its nega ...
.
History
In 1866,Georges Leclanché
Georges Leclanché (9 October 1839 – 14 September 1882) was a French electrical engineer chiefly remembered for his invention of the Leclanché cell, one of the first modern electrical batteries and the forerunner of the modern dry cell battery ...
invented a battery that consisted of a zinc anode and a manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cel ...
cathode wrapped in a porous material, dipped in a jar of ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts. This cell achieved very quick success in telegraphy, signalling and electric bell work.
The dry cell
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its nega ...
form was used to power early telephones—usually from an adjacent wooden box affixed to the wall—before telephones could draw power from the telephone line itself. The Leclanché cell could not provide a sustained current for very long; in lengthy conversations, the battery would run down, rendering the conversation inaudible. This is because certain chemical reactions in the cell increase its internal resistance
In electrical engineering, a practical electric power source which is a linear circuit may, according to Thévenin's theorem, be represented as an ideal voltage source in series with an impedance. This impedance is termed the internal resis ...
and, thus, lower its voltage. These reactions reverse themselves when the battery is left idle, making it good for many short periods of use with idle time between them, but not long periods of use.
Construction
The original form of the cell used a porous pot. This gave it a relatively high internal resistance, and various modifications were made to reduce the resistance. These included the "Agglomerate block cell" and the "Sack cell". Leclanché first, and Carl Gassner later, both strived to transform the original wet cell into a more portable and more efficientdry cell
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its nega ...
.
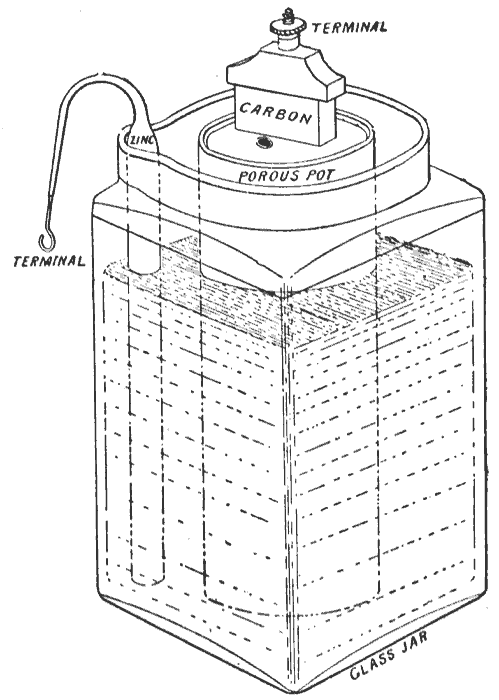
; Porous pot cell: In Leclanché's original cell the depolarizer (in fact, the oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
in the cell), consisting of crushed manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cel ...
, is packed into a pot, and a carbon rod is inserted to act as the cathode (reduction reaction). The anode (oxidation reaction), which is a zinc rod, is then immersed along with the pot in a solution of ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
. The liquid solution acts as the electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
, permeating through the porous pot to make contact with the cathode.
; Agglomerate block cell: In 1871 Leclanché dispensed with the porous pot and replaced it with a pair of "agglomerate blocks", attached to the carbon plate by rubber bands. These blocks were made by mixing the manganese dioxide with binding agents and pressing the mixture into moulds.
; Sack cell: In this cell the porous pot is replaced by a wrapping of canvas or sacking. In addition, the zinc rod is replaced by a zinc cylinder to give a larger surface area. It has a lower internal resistance than either of the above (porous and agglomerate).
; Starch addition: In 1876, Georges Leclanché
Georges Leclanché (9 October 1839 – 14 September 1882) was a French electrical engineer chiefly remembered for his invention of the Leclanché cell, one of the first modern electrical batteries and the forerunner of the modern dry cell battery ...
added starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diet ...
to the ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
electrolyte in an effort to better jellify it.
; Improved dry cell: In 1888, a German physician, Carl Gassner, improved the jellification process and produced a more portable dry cell
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its nega ...
by mixing plaster
Plaster is a building material used for the protective or decorative coating of walls and ceilings and for moulding and casting decorative elements. In English, "plaster" usually means a material used for the interiors of buildings, while "re ...
and hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are n ...
chemicals with the ammonium chloride electrolyte.
Chemistry
The redox reaction in a Leclanché cell involves the two following half-reactions: :–anode
An anode usually is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, which is usually an electrode of the device through which conventional current leaves the devic ...
(oxidation of Zn): Zn → Zn2+ + 2e− , E0 = −0.76 volts
:– cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
(reduction of Mn(IV)): 2 MnO2 + 2NH4+ + 2e− → 2 MnO(OH) + 2 NH3 , E0 = 1.23 volts
The chemical process which produces electricity in a Leclanché cell begins when zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
atoms on the surface of the anode oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
, i.e. they give up both their valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s to become positively charged Zn2+ ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. As the Zn2+ ions move away from the anode, leaving their electrons on its surface, the anode becomes more negatively charged than the cathode. When the cell is connected in an external electrical circuit
An electrical network is an interconnection of electrical components (e.g., battery (electricity), batteries, resistors, inductors, capacitors, switches, transistors) or a model of such an interconnection, consisting of electrical elements (e. ...
, the excess electrons on the zinc anode flow through the circuit to the carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
rod, the movement of electrons forming an electric current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge c ...
. The potential difference
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge ...
in charge over the anode and cathode is equal to the difference of the two half-reaction potentials, producing a theoretical voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
of 1.99v of potential energy across the terminals. A variety of factors, such as internal resistance
In electrical engineering, a practical electric power source which is a linear circuit may, according to Thévenin's theorem, be represented as an ideal voltage source in series with an impedance. This impedance is termed the internal resis ...
, lower this output value to the 1.4 volts measured from these cells in practice.
As the current travels around the circuit, when the electrons enter the cathode (carbon rod), they combine with manganese dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cel ...
(MnO2) and water (H2O), which react with each other to produce manganese oxide (Mn2O3) and negatively charged hydroxide ion
Hydroxide is a polyatomic ion, diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually Self-ionization ...
s. This is accompanied by a secondary acid-base reaction in which the hydroxide ions (OH–) accept a proton (H+) from the ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
ions present in the ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
to produce molecules of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
and water.
:Zn(s) + 2 MnO2(s) + 2 NH4Cl(aq) → ZnCl2(aq) + Mn2O3(s) + 2 NH3(aq) + H2O(l),
or if one also considers the hydration of the Mn2O3(s) sesquioxide into Mn(III) oxy-hydroxide:
:Zn(s) + 2 MnO2(s) + 2 NH4Cl(aq) → ZnCl2(aq) + 2 MnO(OH)(s) + 2 NH3(aq)
Alternately, the reduction reaction of Mn(IV) can proceed further, forming Mn(II) hydroxide. :Zn(s) + MnO2(s) + 2 NH4Cl(aq) → ZnCl2(aq) + Mn(OH)2(s) + 2 NH3(aq)
Uses
Theelectromotive force
In electromagnetism and electronics, electromotive force (also electromotance, abbreviated emf, denoted \mathcal) is an energy transfer to an electric circuit per unit of electric charge, measured in volts. Devices called electrical ''transducer ...
(e.m.f.) produced by a Leclanche cell is 1.4 volt
The volt (symbol: V) is the unit of electric potential, Voltage#Galvani potential vs. electrochemical potential, electric potential difference (voltage), and electromotive force in the International System of Units, International System of Uni ...
s, with a resistance of several ohm
Ohm (symbol Ω) is a unit of electrical resistance named after Georg Ohm.
Ohm or OHM may also refer to:
People
* Georg Ohm (1789–1854), German physicist and namesake of the term ''ohm''
* Germán Ohm (born 1936), Mexican boxer
* Jörg Ohm (1 ...
s where a porous pot is used. It saw extensive usage in telegraphy
Telegraphy is the long-distance transmission of messages where the sender uses symbolic codes, known to the recipient, rather than a physical exchange of an object bearing the message. Thus flag semaphore is a method of telegraphy, whereas pi ...
, signaling, electric bells and similar applications where intermittent current was required and it was desirable that a battery should require little maintenance.
The Leclanché battery wet cell was the forerunner of the modern zinc–carbon battery (a dry cell
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its nega ...
). The addition of zinc chloride
Zinc chloride is an Inorganic chemistry, inorganic chemical compound with the chemical formula, formula ZnCl2·''n''H2O, with ''n'' ranging from 0 to 4.5, forming water of hydration, hydrates. Zinc chloride, anhydrous and its hydrates, are colo ...
to the electrolyte paste raises the e.m.f. to 1.5 volts. Later developments dispensed with the ammonium chloride completely, giving a cell that can endure more sustained discharge without its internal resistance rising as quickly (the zinc chloride cell).
See also
*List of battery types
This list is a summary of notable electric battery types composed of one or more electrochemical cells. Three lists are provided in the table. The primary (non-rechargeable) and secondary (rechargeable) cell lists are lists of battery chemistry. ...
* History of the battery
* Alkaline battery
An alkaline battery (IEC code: L) is a type of primary battery where the electrolyte (most commonly potassium hydroxide) has a pH value above 7. Typically, these batteries derive energy from the reaction between zinc metal and manganese diox ...
, a similar cell in which the NH4Cl electrolyte has been replaced by KOH. This improved type of battery with a much higher charge density (5 ×) was commercialised much later (1960/70).
References
Bibliography
* ''Practical Electricity'' by W. E. Ayrton and T. Mather, published by Cassell and Company, London, 1911, pp 188–193 {{Galvanic cells Wet cell batteries 1866 in science French inventions