|
Thermodynamic Modelling
Thermodynamic modelling is a set of different strategies that are used by engineers and scientists to develop models capable of evaluating different List of thermodynamic properties, thermodynamic properties of a system. At each thermodynamic equilibrium state of a system, the List of thermodynamic properties, thermodynamic properties of the system are specified. Generally, thermodynamic models are mathematical relations that relate different state properties to each other in order to eliminate the need of measuring all the properties of the system in different states. The easiest thermodynamic models, also known as equation of state, equations of state, can come from simple correlations that relate different thermodynamic properties using a linear or second-order polynomial function of temperature and pressures. They are generally fitted using experimental data available for that specific properties. This approach can result in limited predictability of the correlation and as a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Thermodynamic Properties
In thermodynamics, a physical property is any property that is measurable, and whose value describes a state of a physical system. Thermodynamic properties are defined as characteristic features of a system, capable of specifying the system's state. Some constants, such as the ideal gas constant, , do not describe the state of a system, and so are not properties. On the other hand, some constants, such as (the freezing point depression constant, or cryoscopic constant), depend on the identity of a substance, and so may be considered to describe the state of a system, and therefore may be considered physical properties. "Specific" properties are expressed on a per mass basis. If the units were changed from per mass to, for example, per mole, the property would remain as it was (i.e., Intensive and extensive properties, intensive or extensive). Regarding work and heat Work (thermodynamics), Work and heat are not thermodynamic properties, but rather ''process function, process qu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
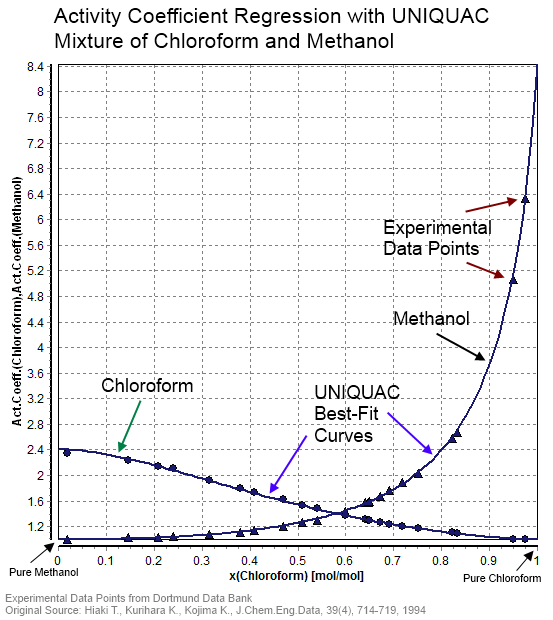
UNIQUAC
In statistical thermodynamics, UNIQUAC (a portmanteau of universal quasichemical) is an activity coefficient model used in description of phase equilibria. The model is a so-called lattice model and has been derived from a first order approximation of interacting molecule surfaces. The model is, however, not fully thermodynamically consistent due to its two- liquid mixture approach. In this approach the local concentration around one central molecule is assumed to be independent from the local composition around another type of molecule. The UNIQUAC model can be considered a second generation activity coefficient because its expression for the excess Gibbs energy consists of an entropy term in addition to an enthalpy term. Earlier activity coefficient models such as the Wilson equation and the non-random two-liquid model (NRTL model) only consist of enthalpy terms. Today the UNIQUAC model is frequently applied in the description of phase equilibria (i.e. liquid–solid, liqui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Models
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the first to formulate a concise d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Combining Rules
In computational chemistry and molecular dynamics, the combination rules or combining rules are equations that provide the interaction energy between two dissimilar non-bonded atoms, usually for the part of the potential representing the van der Waals interaction. In the simulation of mixtures, the choice of combining rules can sometimes affect the outcome of the simulation. Combining rules for the Lennard-Jones potential The Lennard-Jones Potential is a mathematically simple model for the interaction between a pair of atoms or molecules. One of the most common forms is : V_ = 4\varepsilon \left \left(\frac\right)^ - \left(\frac\right)^ \right where ''ε'' is the depth of the potential well, ''σ'' is the finite distance at which the inter-particle potential is zero, ''r'' is the distance between the particles. The potential reaches a minimum, of depth ''ε'', when ''r'' = 21/6σ ≈ 1.122σ. Lorentz-Berthelot rules The Lorentz rule was proposed by H. A. Lorentz in 1881: :\sigma_ = ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equation Of State
In physics and chemistry, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Though there are many equations of state, none accurately predicts properties of substances under all conditions. The quest for a universal equation of state has spanned three centuries. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of mass nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, not only is there an absence of macroscopic change, but there is an “absence of any ''tendency'' toward change on a macroscopic scale.” Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, while not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, unless disturbed by a thermodynamic operation. In a macroscopic equilibrium, perfectly or almost perfectly ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Order Phase Transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition States of matter Phase transitions commonly refer to when a substance transforms between one of the four states of matter to an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature ( isothermal). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium. In contrast, the Gibbs free energy or free enthalpy is most commonly used as a measure of thermodynamic potential (especially in chemistry) when it is convenient for applications that occur at constant ''pressure''. For example, in explosives research Helmholtz free energy is often used, since explosive reactions by their nature induce pressure changes. It is also frequently used to define fundamental equations of state of pure substances. The concept of free energy was developed by Hermann von Helmholtz, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
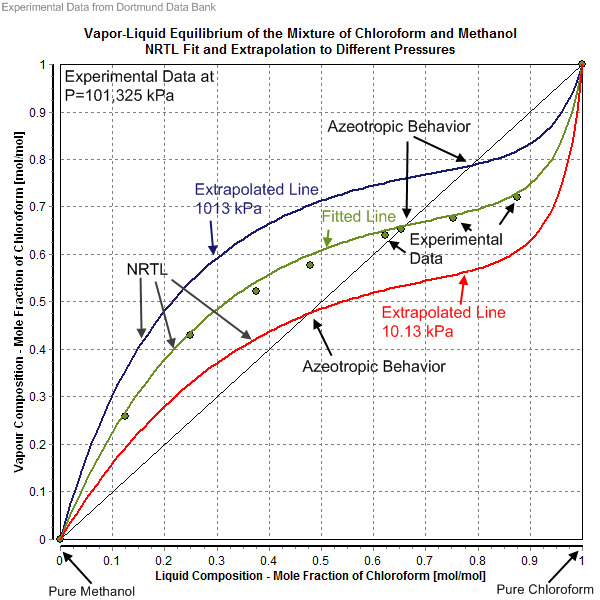
Non-random Two-liquid Model
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model introduced by Renon and John Prausnitz, Prausnitz in 1968 that correlates the activity coefficients \gamma_i of a compound with its mole fractions x_i in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson, who stated that the local concentration around a molecule in most mixtures is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind U_ and that with the molecules of the other kind U_. The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC model, and the group contribution model UNIFAC. These local-com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wong–Sandler Mixing Rule
The Wong–Sandler mixing rule is a thermodynamic mixing rule used for vapor–liquid equilibrium and liquid-liquid equilibrium calculations. __TOC__ Summary The first boundary condition is :b - \frac a = \sum_i \sum_j x_i x_j \left(b_ - \frac \right) which constrains the sum of ''a'' and ''b''. The second equation is :\underline A^_(T, P\to\infty, \underline x) = \underline A^_(T, P\to\infty, \underline x) with the notable limit as P\to \infty (and \underline_i\to b, \underline_\to b) of :\underline A^_ = C^* \left(\frac a b - \sum x_i \frac \right). The mixing rules become :\frac = Q \frac,\quad b = \frac :Q = \sum_i \sum_j x_i x_j \left( b_ - \frac \right) :D = \sum_i x_i \frac + \frac The cross term still must be specified by a combining rule, either :b_ - \frac = \sqrt (1 - k_) or :b_ - \frac = \frac(b_ + b_) - \frac(1 - k_). See also :Vapor–liquid equilibrium :Equation of state In physics and chemistry, an equation of state is a thermodynamic equation rela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Logic Chart For Cubic Thermodynamic Model Development Of Mixtures
Logic is the study of correct reasoning. It includes both formal and informal logic. Formal logic is the study of deductively valid inferences or logical truths. It examines how conclusions follow from premises based on the structure of arguments alone, independent of their topic and content. Informal logic is associated with informal fallacies, critical thinking, and argumentation theory. Informal logic examines arguments expressed in natural language whereas formal logic uses formal language. When used as a countable noun, the term "a logic" refers to a specific logical formal system that articulates a proof system. Logic plays a central role in many fields, such as philosophy, mathematics, computer science, and linguistics. Logic studies arguments, which consist of a set of premises that leads to a conclusion. An example is the argument from the premises "it's Sunday" and "if it's Sunday then I don't have to work" leading to the conclusion "I don't have to work." Premise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", ''AIChE Journal'', vol. 21 (1975), p. 1086 is a semi-empirical system for the prediction of non-electrolyte activity in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients. By using interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain information on liquid equilibria, which is useful in many thermodynamic calculations, such as chemical reactor design, and distillation calculations. The UNIFAC model was first published in 1975 by Fredenslund, Jones and John Prausnitz, a group of chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |