|
Strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see strontian proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium 1
Strontium is a chemical element; it has Symbol (chemistry), symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly Reactivity (chemistry), chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals Celestine (mineral), celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank (chemist), William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, stron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline Earth Metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all shiny, silvery-white, somewhat reactivity (chemistry), reactive metals at standard temperature and pressure. Together with helium, these elements have in common an outer atomic orbital, s orbital which is full —that is, this orbital contains its full complement of two electrons, which the alkaline earth metals readily lose to form cations with electric charge, charge +2, and an oxidation state of +2. Helium is grouped with the noble gases and not with the alkaline earth metals, but it is theorized to have some similarities to beryllium when forced into bonding and has sometimes been suggested to belong to group 2. All the discovered alkaline earth metals occur in nature, although radium occurs only through the d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.79 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and industry and is an isotope of concern in fallout from nuclear weapons, nuclear weapons testing, and nuclear accidents. Radioactivity Naturally occurring strontium is nonradioactive and nontoxic at levels normally found in the environment, but 90Sr is a radiation hazard. 90Sr undergoes β− decay with a half-life of 28.79 years and a decay energy of 0.546 MeV distributed to an electron, an antineutrino, and the yttrium isotope 90Y, which in turn undergoes β− decay with a half-life of 64 hours and a decay energy of 2.28 MeV distributed to an electron, an antineutrino, and 90Zr (zirconium), which is stable. Note that 90Sr/Y is almost a pure beta particle source; the gamma photon emission from the decay of 90Y is so infrequen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Oxide
Strontium oxide or strontia, SrO, is formed when strontium reacts with oxygen. Burning strontium in air results in a mixture of strontium oxide and strontium nitride. It also forms from the decomposition of strontium carbonate SrCO3. It is a strongly basic oxide. Uses About 8% by weight of cathode-ray tubes is strontium oxide, which has been the major use of strontium since 1970. Color televisions and other devices containing color cathode-ray tubes sold in the United States are required by law to use strontium in the faceplate to block X-ray emission (these X-ray emitting TVs are no longer in production). Lead(II) oxide can be used in the neck and funnel, but causes discoloration when used in the faceplate. Reactions Elemental strontium is formed when strontium oxide is heated with aluminium Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of othe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Hydroxide
Strontium hydroxide, Sr(OH)2, is a caustic alkali composed of one strontium ion and two hydroxide ions. It is synthesized by combining a strontium Salt (chemistry), salt with a strong base. Sr(OH)2 exists in anhydrous, hydrate, monohydrate, or octahydrate form. Preparation Because Sr(OH)2 is slightly soluble in cold water, its preparation can be easily carried out by the addition of a strong base such as Sodium hydroxide, NaOH or Potassium hydroxide, KOH, drop by drop to a solution of any soluble strontium salt, most commonly Sr(NO3)2 (strontium nitrate). The Sr(OH)2 will precipitate out as a fine white powder. From here, the solution is filtered, and the Sr(OH)2 is washed with cold water and dried. Applications Strontium hydroxide is used chiefly in the refining of beet sugar and as a stabilizer in plastic. It may be used as a source of strontium ions when the chlorine from strontium chloride is undesirable. Strontium hydroxide absorbs carbon dioxide from the air to form stron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontianite
Strontianite (Strontium, SrCarbon, COxygen, O3) is an important raw material for the extraction of strontium. It is a rare carbonate mineral and one of only a few strontium minerals. It is a member of the aragonite group. Aragonite group members: aragonite (CaCO3), witherite (BaCO3), strontianite (SrCO3), cerussite (PbCO3) The ideal formula of strontianite is SrCO3, with molar mass 147.63 g, but calcium (Ca) can substitute for up to 27% of the strontium (Sr) Ion, cations, and barium (Ba) up to 3.3%. The mineral was named in 1791 for the locality, Strontian, Argyllshire, Scotland, where the element strontium had been discovered the previous year. Although good mineral specimens of strontianite are rare, strontium is a fairly common Chemical element, element, with abundance in the Crust (geology), Earth's crust of 370 parts per million by weight, 87 parts per million by Mole (unit), moles, much more common than copper with only 60 parts per million by weight, 19 by moles. Stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celestine (mineral)
Celestine (the IMA-accepted name) or celestite is a mineral consisting of strontium sulfate ( Sr S O). The mineral is named for its occasional delicate blue color. Celestine and the carbonate mineral strontianite are the principal sources of the element strontium, commonly used in fireworks and in various metal alloys. Etymology Celestine derives its name from the Latin word ''caelestis'' meaning celestial which in turn is derived from the Latin word ''caelum'' meaning sky, air, weather, atmosphere and heaven. Occurrence Celestine occurs as crystals, and also in compact massive and fibrous forms. It is mostly found in sedimentary rocks, often associated with the minerals gypsum, anhydrite, and halite. On occasion in some localities, it may also be found with sulfur inclusions. The mineral is found worldwide, usually in small quantities. Pale blue crystal specimens are found in Madagascar. White and orange variants also occurred at Yate, Bristol, UK, where it was ext ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
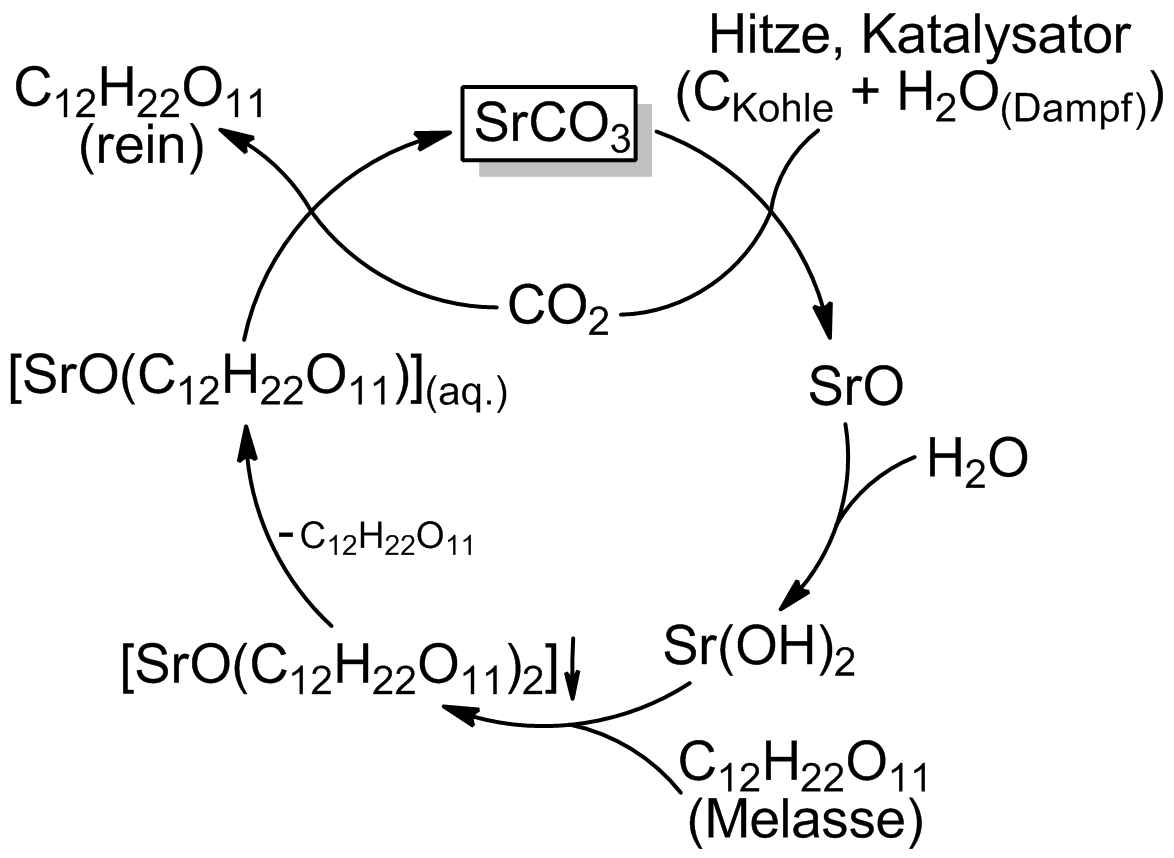
Strontian Process
The strontian process is an obsolete chemical method to recover sugar from molasses. Its use in Europe peaked in the middle of the 19th century. The name ''strontian'' comes from the Scottish village Strontian where the source mineral strontianite (strontium carbonate) was first found. Chemistry Strontium carbonate is a recycled coreactant in this process. # Strontium carbonate is calcination, calcined with carbon in the presence of steam to form strontium hydroxide. The strontium and carbon dioxide formed are rejoined later in the process, forming strontium carbonate once again. #: SrCO3 + C + H2O + O2 = Sr(OH)2 + 2 CO2 # In a molasses solution kept near 100 °C, the hydroxide reacts with soluble sugars to form water and the poorly soluble strontium saccharide which is filtered out, but kept awash in near-boiling water. #: Sr(OH)2 + 2C12H22O11 = SrO(C12H22O11)2 + H2O # The saccharate liquid is cooled to 10 °C, cracking off one of the sugars #: SrO(C12H22O11)2 = SrO(C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode-ray Tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a Film frame, frame of video on an Analog television, analog television set (TV), Digital imaging, digital raster graphics on a computer monitor, or other phenomena like radar targets. A CRT in a TV is commonly called a picture tube. CRTs have also been Williams tube, used as memory devices, in which case the screen is not intended to be visible to an observer. The term ''cathode ray'' was used to describe electron beams when they were first discovered, before it was understood that what was emitted from the cathode was a beam of electrons. In CRT TVs and computer monitors, the entire front area of the tube is scanned repeatedly and systematically in a fixed pattern called a raster scan, raster. In color devices, an image is produced by con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontian
Strontian (; ) is the main village in Sunart, an area in western Lochaber, Scottish Highlands, Highland, Scotland, on the A861 road. Prior to 1975 it was part of Argyllshire. It lies on the north shore of Loch Sunart, close to the head of the loch. In the hills to the north of Strontian lead was mined in the 18th century and in these mines the mineral strontianite was discovered, from which the Chemical element, element strontium was first isolated. The village name in Scots Gaelic, Gaelic, ''Sròn an t-Sìthein'', translates as the ''nose'' [i.e. 'point'] ''of the fairy hill'', meaning a knoll or low round hill inhabited by the mythological ''sídhe''. The nearby hamlets of Anaheilt, Bellsgrove, and Upper and Lower Scotstown are now generally considered part of Strontian, with Polloch several miles away on the terminus of the road to Loch Shiel. Strontian is the location of Ardnamurchan High School, the local fire station, police station and other facilities. Geology and m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. The most common minerals of barium are barite ( barium sulfate, BaSO4) and witherite ( barium carbonate, BaCO3). The name ''barium'' originates from the alchemical derivative "baryta", from Greek (), meaning 'heavy'. ''Baric'' is the adjectival form of barium. Barium was identified as a new element in 1772, but not reduced to a metal until 1808 with the advent of electrolysis. Barium has few industrial applications. Historically, it was used as a getter for vacuum tubes and in oxide form as the emissive coating on indirectly heated cathodes. It is a component of YBCO (high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. Barium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, electricity and thermal conductivity, heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against nonmetallic materials which do not. Metals are typically ductile (can be drawn into a wire) and malleable (can be shaped via hammering or pressing). A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polythiazyl, polymeric sulfur nitride. The general science of metals is called metallurgy, a subtopic of materials science; aspects of the electronic and thermal properties are also within the scope of condensed matter physics and solid-state chemistry, it is a multidisciplinary topic. In colloquial use materials such as steel alloys are referred to as metals, while others such as polymers, wood or ceramics are nonmetallic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |