|
Steam Boiler 3 English
Steam is water vapor, often mixed with air or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Saturated or superheated steam is invisible; however, wet steam, a visible mist or aerosol of water droplets, is often referred to as "steam". When liquid water becomes steam, it increases in volume by 1,700 times at standard temperature and pressure; this change in volume can be converted into mechanical work by steam engines such as reciprocating piston type engines and steam turbines, which are a sub-group of steam engines. Piston type steam engines played a central role in the Industrial Revolution and modern steam turbines are used to generate more than 80% of the world's electricity. If liquid water comes in contact with a very hot surface or depressurizes quickly below its vapour pressure, it can create a steam explosion. Types of steam and conversions Steam is trad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Explosion
A steam explosion is an explosion caused by violent boiling or flashing of water or ice into steam, occurring when water or ice is either superheated, rapidly heated by fine hot debris produced within it, or heated by the interaction of molten metals (as in a fuel–coolant interaction, or FCI, of molten nuclear-reactor fuel rods with water in a nuclear reactor core following a core-meltdown). Steam explosions are instances of explosive boiling. Pressure vessels, such as pressurized water (nuclear) reactors, that operate above atmospheric pressure can also provide the conditions for a steam explosion. The water changes from a solid or liquid to a gas with extreme speed, increasing dramatically in volume. A steam explosion sprays steam and boiling-hot water and the hot medium that heated it in all directions (if not otherwise confined, e.g. by the walls of a container), creating a danger of scalding and burning. Steam explosions are not normally chemical explosions, althou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soil Steam Sterilization
Soil steam sterilization (soil steaming) is a farming technique that Sterilization (microbiology), sterilizes soil with steam in open fields or greenhouses. Pests of plant cultures such as weeds, bacteria, fungi and viruses are killed through induced hot steam which causes vital cellular proteins to Protein folding, unfold. Biologically, the method is considered a partial disinfection. Important heat-resistant, spore-forming bacteria can survive and revitalize the soil after cooling down. Soil fatigue can be cured through the release of nutritive substances blocked within the soil. Steaming leads to a better starting position, quicker growth and strengthened resistance against plant disease and pests. Today, the application of hot steam is considered the best and most effective way to disinfect sick soil, potting soil and compost. It is being used as an alternative to bromomethane, whose production and use was curtailed by the Montreal Protocol. "Steam effectively kills pathog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agriculture
Agriculture encompasses crop and livestock production, aquaculture, and forestry for food and non-food products. Agriculture was a key factor in the rise of sedentary human civilization, whereby farming of domesticated species created food surpluses that enabled people to live in the cities. While humans started gathering grains at least 105,000 years ago, nascent farmers only began planting them around 11,500 years ago. Sheep, goats, pigs, and cattle were domesticated around 10,000 years ago. Plants were independently cultivated in at least 11 regions of the world. In the 20th century, industrial agriculture based on large-scale monocultures came to dominate agricultural output. , small farms produce about one-third of the world's food, but large farms are prevalent. The largest 1% of farms in the world are greater than and operate more than 70% of the world's farmland. Nearly 40% of agricultural land is found on farms larger than . However, five of every six farm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
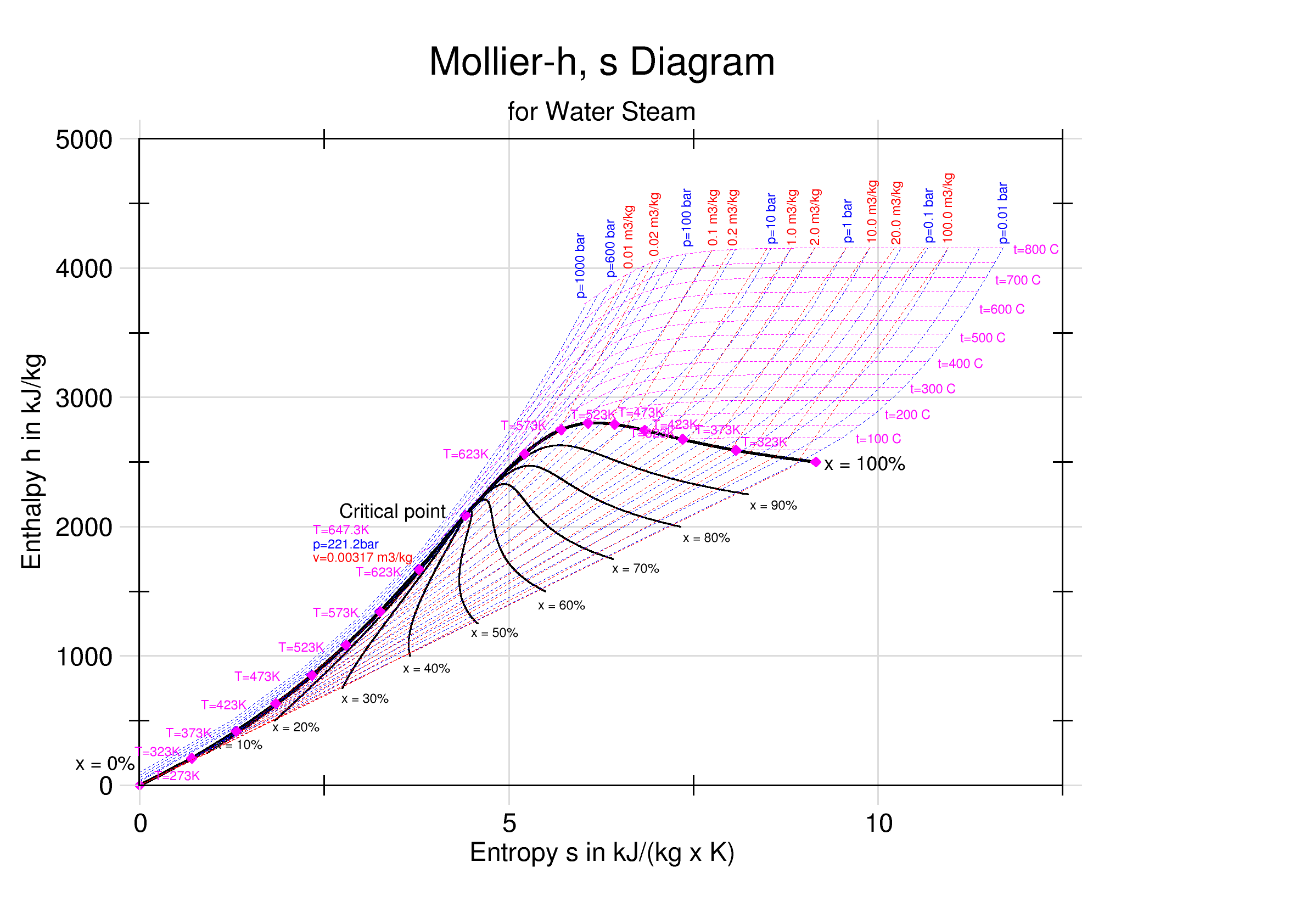
Mollier Enthalpy Entropy Chart For Steam - US Units
Mollier is a surname. People with the surname include: * Dominique Mollier (born 1949), French freestyle swimmer * Jean-Yves Mollier (born 1947), French Contemporary History teacher * Louis-Marie Mollier (1846–1911), French-American pioneer priest of north-central Kansas * Pierre Mollier (born 1961), French historian and freemason * Richard Mollier (1863–1935), German professor of applied physics and mechanics {{surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy–entropy Chart
An enthalpy–entropy chart, also known as the ''H''–''S'' chart or Mollier diagram, plots the total heat against entropy, describing the enthalpy of a thermodynamic system. A typical chart covers a pressure range of 0.01–1000 Bar (unit), bar, and temperatures up to 800 degrees Celsius. It shows enthalpy H in terms of internal energy U, pressure p and volume V using the relationship H = U + pV \,\! (or, in terms of specific enthalpy, specific entropy and specific volume, h = u + pv \! ). History The diagram was created in 1904, when Richard Mollier plotted the total heat against entropy . At the 1923 Thermodynamics Conference held in Los Angeles it was decided to name, in his honor, as a "Mollier diagram" any thermodynamic diagram using the enthalpy as one of its axes. Details On the diagram, lines of constant pressure, constant temperature and volume are plotted, so in a two-phase region, the lines of constant pressure and temperature coincide. Thus, coordinates on t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Table
This page provides supplementary data to the article properties of water. Further comprehensive authoritative data can be found at the ''NIST Chemistry WebBook'' page on thermophysical properties of fluids. Structure and properties Thermodynamic properties Liquid physical properties Water/steam equilibrium properties Vapor pressure formula for steam in equilibrium with liquid water: : \log_ P = A - \frac, where ''P'' is equilibrium vapor pressure in k Pa, and ''T'' is temperature in kelvins. For ''T'' = 273 K to 333 K: ''A'' = 7.2326; ''B'' = 1750.286; ''C'' = 38.1. For ''T'' = 333 K to 423 K: ''A'' = 7.0917; ''B'' = 1668.21; ''C'' = 45.1. Data in the table above is given for water–steam equilibria at various temperatures over the entire temperature range at which liquid water can exist. Pressure of the equilibrium is given in the second column in k Pa. The third column is the heat content of each gram of the liquid phase relative to water at 0 °C. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spirax-Sarco Engineering
Spirax Group plc, formerly Spirax-Sarco Engineering plc, is a British manufacturer of steam management systems and peristaltic pumps and associated fluid path technologies. It is headquartered in Cheltenham, England. It is listed on the London Stock Exchange and is a constituent of the FTSE 100 Index. History The company was founded by Herman Sanders in 1888 and after a Mr Rehders joined the business, established as ''Sanders, Rehders & Co.'' ('Sarco') in London. Initially, it focused upon the importing of thermostatic steam traps from Germany. During 1932, Sarco commenced the domestic manufacture of steam traps under the ''Spirax'' brand name. Between 1952 and 1968, the business was headed by Lionel Northcroft. In 1959, it was listed on the London Stock Exchange as ''Spirax-Sarco Engineering''. During 1960, Spirax-Sarco launched its first range of self-acting pressure controls. Three years later, it acquired ''Drayton Controls'', a rival control valve and instrumentation bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: ) under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boiling, boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one Atmosphere (unit), atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |