Enthalpy–entropy chart on:
[Wikipedia]
[Google]
[Amazon]
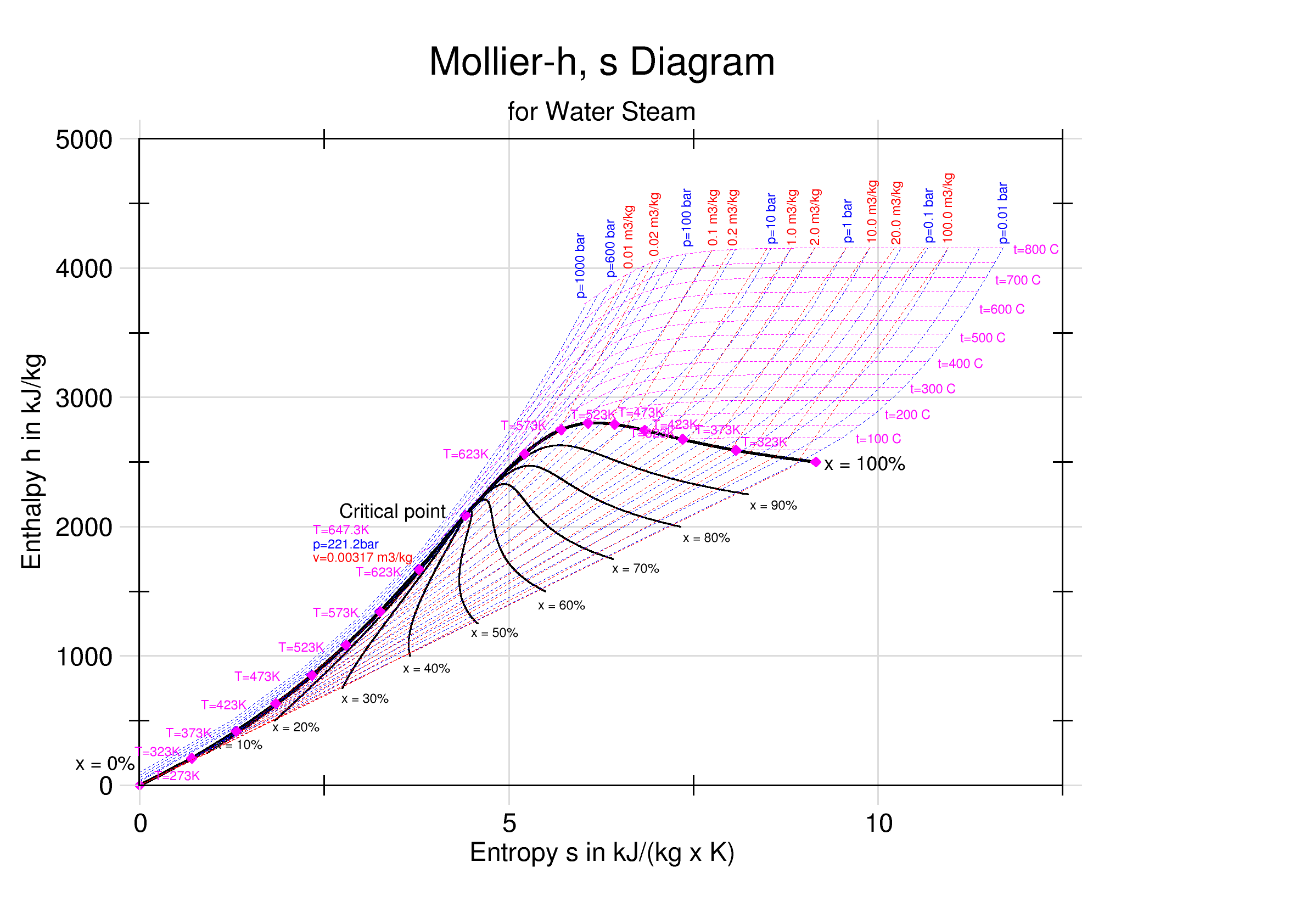 An enthalpy–entropy chart, also known as the ''H''–''S'' chart or Mollier diagram, plots the total heat against entropy, describing the
An enthalpy–entropy chart, also known as the ''H''–''S'' chart or Mollier diagram, plots the total heat against entropy, describing the
 An enthalpy–entropy chart, also known as the ''H''–''S'' chart or Mollier diagram, plots the total heat against entropy, describing the
An enthalpy–entropy chart, also known as the ''H''–''S'' chart or Mollier diagram, plots the total heat against entropy, describing the enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
of a thermodynamic system
A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics.
Thermodynamic systems can be passive and active according to internal processes. According to inter ...
. A typical chart covers a pressure range of 0.01–1000 bar, and temperatures up to 800 degrees Celsius
The degree Celsius is the unit of temperature on the Celsius temperature scale "Celsius temperature scale, also called centigrade temperature scale, scale based on 0 ° for the melting point of water and 100 ° for the boiling point ...
. It shows enthalpy in terms of internal energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accoun ...
, pressure and volume using the relationship (or, in terms of specific enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
, specific entropy and specific volume
In thermodynamics, the specific volume of a substance (symbol: , nu) is the quotient of the substance's volume () to its mass ():
:\nu = \frac
It is a mass-specific intrinsic property of the substance. It is the reciprocal of density (rho) ...
, ).
History
The diagram was created in 1904, when Richard Mollier plotted the total heat against entropy . At the 1923 Thermodynamics Conference held in Los Angeles it was decided to name, in his honor, as a "Mollier diagram" any thermodynamic diagram using the enthalpy as one of its axes.Details
On the diagram, lines of constant pressure, constant temperature and volume are plotted, so in a two-phase region, the lines of constant pressure and temperature coincide. Thus, coordinates on the diagram represententropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the micros ...
and heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
.
The work done
In science, work is the energy transferred to or from an object via the application of force along a displacement. In its simplest form, for a constant force aligned with the direction of motion, the work equals the product of the force stren ...
in a process on vapor cycles is represented by length of , so it can be measured directly, whereas in a T–s diagram it has to be computed using thermodynamic relationship between thermodynamic properties.
In an isobaric process
In thermodynamics, an isobaric process is a type of thermodynamic process in which the pressure of the Thermodynamic system, system stays constant: Δ''P'' = 0. The heat transferred to the system does work (thermodynamics), work, but a ...
, the pressure remains constant, so the heat interaction is the change in enthalpy.
In an isenthalpic process
An isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, ''H''; or specific enthalpy, ''h''.
Overview
If a steady-state, steady-flow process is analysed using a control volume, everything outside ...
, the enthalpy is constant. A horizontal line in the diagram represents an isenthalpic process.
A vertical line in the ''h–s'' chart represents an isentropic
An isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in eng ...
process. The process 3–4 in a Rankine cycle
The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat sour ...
is isentropic
An isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in eng ...
when the steam turbine
A steam turbine or steam turbine engine is a machine or heat engine that extracts thermal energy from pressurized steam and uses it to do mechanical work utilising a rotating output shaft. Its modern manifestation was invented by Sir Charles Par ...
is said to be an ideal one. So the expansion process in a turbine can be easily calculated using the h–s chart when the process is considered to be ideal (which is the case normally when calculating enthalpies, entropies, etc. Later the deviations from the ideal values and they can be calculated considering the isentropic efficiency of the steam turbine used.)
Lines of constant ''dryness fraction'' (''x''), sometimes called the ''quality'', are drawn in the wet region and lines of constant temperature are drawn in the superheated region. ''X'' gives the fraction (by mass) of gaseous substance in the wet region, the remainder being colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others exte ...
al liquid droplets. Above the heavy line, the temperature is above the boiling point, and the dry (superheated) substance is gas only.
In general such charts do not show the values of specific volume
In thermodynamics, the specific volume of a substance (symbol: , nu) is the quotient of the substance's volume () to its mass ():
:\nu = \frac
It is a mass-specific intrinsic property of the substance. It is the reciprocal of density (rho) ...
s, nor do they show the enthalpies of saturated water at pressures which are of the order of those experienced in condensers in a thermal power station
A thermal power station, also known as a thermal power plant, is a type of power station in which the heat energy generated from various fuel sources (e.g., coal, natural gas, nuclear fuel, etc.) is converted to electrical energy. The heat ...
. Hence the chart is only useful for enthalpy changes in the expansion process of the steam cycle.
Applications and usage
It can be used in practical applications such asmalt
Malt is any cereal grain that has been made to germinate by soaking in water and then stopped from germinating further by drying with hot air, a process known as "malting".
Malted grain is used to make beer, whisky, malted milk, malt vinegar, ...
ing, to represent the grain–air–moisture system.
The underlying property data for the Mollier diagram is identical to a psychrometric chart
Psychrometrics (or psychrometry, ; also called hygrometry) is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.
History
With the inventions of the hygrometer and thermometer, the theori ...
. At first inspection, there may appear little resemblance between the charts, but if the user rotates a chart ninety degrees and looks at it in a mirror, the resemblance is apparent. The Mollier diagram coordinates are enthalpy ''h'' and humidity ratio ''x''. The enthalpy coordinate is ''skewed'' and the constant enthalpy lines are parallel and evenly spaced.
See also
*Thermodynamic diagrams
Thermodynamic diagrams are diagrams used to represent the thermodynamic states of a material (typically fluid) and the consequences of manipulating this material. For instance, a temperature–entropy diagram (Temperature–entropy diagram, T–s ...
*Contour line
A contour line (also isoline, isopleth, isoquant or isarithm) of a Function of several real variables, function of two variables is a curve along which the function has a constant value, so that the curve joins points of equal value. It is a ...
* Phase diagram
References
{{DEFAULTSORT:Enthalpy-entropy chart Thermodynamics Entropy de:Wasserdampf#h-s-Diagramm