|
RXNO Ontology
The RXNO Ontology is a formal ontology (information science), ontology of chemical name reaction, named reactions. It was originally developed at the Royal Society of Chemistry (RSC) and is associated with the Open Biomedical Ontologies Foundry. The RXNO ontology unifies several previous attempts to systematize chemical reactions including the Merck Index and the hierarchy of Carey, Laffan, Thomson and Williams. Major Reaction Categories The twelve top-level reaction categories proposed by Carey, Laffan, Thompson and Williams are given in the table below, together with their RXNO ontology identifiers and the equivalent Wikipedia categories where applicable. Name Reactions The following table lists the RXNO identifiers for some example name reactions. :RXNO:0000003 Perkin reaction :RNXO:0000006 Diels–Alder reaction :RXNO:0000014 Grignard reaction :RXNO:0000015 Wittig reaction :RXNO:0000021 Sandmeyer reaction :RXNO:0000024 Heck reaction :RXNO:0000026 Beckmann rearrangemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wurtz Reaction
In organic chemistry, the Wurtz reaction, named after Charles Adolphe Wurtz, is a coupling reaction whereby two alkyl halides are treated with sodium metal to form a higher alkane. : 2 R−X + 2 Na → R−R + 2 NaX The reaction is of little value except for intramolecular versions. A related reaction, which combines alkyl halides with aryl halides is called the Wurtz–Fittig reaction. Mechanism The reaction proceeds by an initial metal–halogen exchange, which involves this idealized stoichiometry: : R−X + 2 M → RM + MX This step involves the intermediacy of radical species R·. This step resembles the formation of a Grignard reagent. These RM intermediates have been isolated in several cases. The organometallic intermediate next reacts with the alkyl halide forming a new carbon–carbon covalent bond. : RM + RX → R−R + MX The process resembles an SN2 reaction, but the process is probably complex, which may explain the inefficiency of the reaction. Examp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Indole Synthesis
The Fischer indole synthesis is a chemical reaction that produces the aromatic Heterocyclic compound, heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today migraine, antimigraine drugs of the triptan class are often synthesized by this method. This reaction can be catalyzed by Brønsted acids such as HCl, sulfuric acid, H2SO4, polyphosphoric acid and p-toluenesulfonic acid or Lewis acids such as boron trifluoride, zinc chloride, iron chloride, and aluminium chloride. Several reviews have been published. Reaction mechanism The reaction of a (substituted) phenylhydrazine with a carbonyl (aldehyde or ketone) initially forms a phenylhydrazone which isomerization, isomerizes to the respective enamine (or 'ene-hydrazine'). After protonation, a cyclic sigmatropic rearrangement, [3,3]-sigmatropic rearrangement occurs producing an imine. The resulting imine forms a cyclic amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skraup Reaction
The Skraup synthesis is a chemical reaction used to synthesize quinolines. It is named after the Czech chemist Zdenko Hans Skraup (1850-1910). In the archetypal Skraup reaction, aniline is heated with sulfuric acid, glycerol, and an oxidizing agent such as nitrobenzene to yield quinoline. In this example, nitrobenzene serves as both the solvent and the oxidizing agent. The reaction, which otherwise has a reputation for being violent, is typically conducted in the presence of ferrous sulfate. Arsenic acid Arsenic acid or trihydrogen arsenate is the chemical compound with the formula . More descriptively written as , this colorless acid is the arsenic analogue of phosphoric acid. Arsenate and phosphate salts behave very similarly. Arsenic acid as ... may be used instead of nitrobenzene and the former is better since the reaction is less violent. See also * Bischler-Napieralski reaction * Doebner-Miller reaction References {{DEFAULTSORT:Skraup Reaction Condensati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Horner–Wadsworth–Emmons Reaction
The Horner–Wadsworth–Emmons (HWE) reaction is a chemical reaction used in organic chemistry of stabilized phosphonate carbanions with aldehydes (or ketones) to produce predominantly E-alkenes. In 1958, Leopold Horner published a modified Wittig reaction using phosphonate-stabilized carbanions. William S. Wadsworth and William D. Emmons further defined the reaction. In contrast to phosphonium ylides used in the Wittig reaction, phosphonate-stabilized carbanions are more nucleophilic but less basic. Likewise, phosphonate-stabilized carbanions can be alkylated. Unlike phosphonium ylides, the dialkylphosphate salt byproduct is easily removed by aqueous extraction. Several reviews have been published. Reaction mechanism The Horner–Wadsworth–Emmons reaction begins with the deprotonation of the phosphonate to give the phosphonate carbanion 1. Nucleophilic addition of the carbanion onto the aldehyde 2 (or ketone) producing 3a or 3b is the rate-limiting step. If R2 = H, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β- diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Birch Reduction
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally liquid ammonia) with an alkali metal (traditionally sodium) and a proton source (traditionally an alcohol). Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane. An example is the reduction of naphthalene in ammonia and ethanol: Reaction mechanism and regioselectivity A solution of sodium in liquid ammonia consists of the intensely blue electride salt a(NH3)xsup>+ e−. The solvated electrons add to the aromatic ring to give a radical anion, which then abstracts a proton from the alcohol. The process then repeats at either the ''ortho'' or ''para'' position (depending on substituents) to give the final diene. The residual double bonds do not stabilize further radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
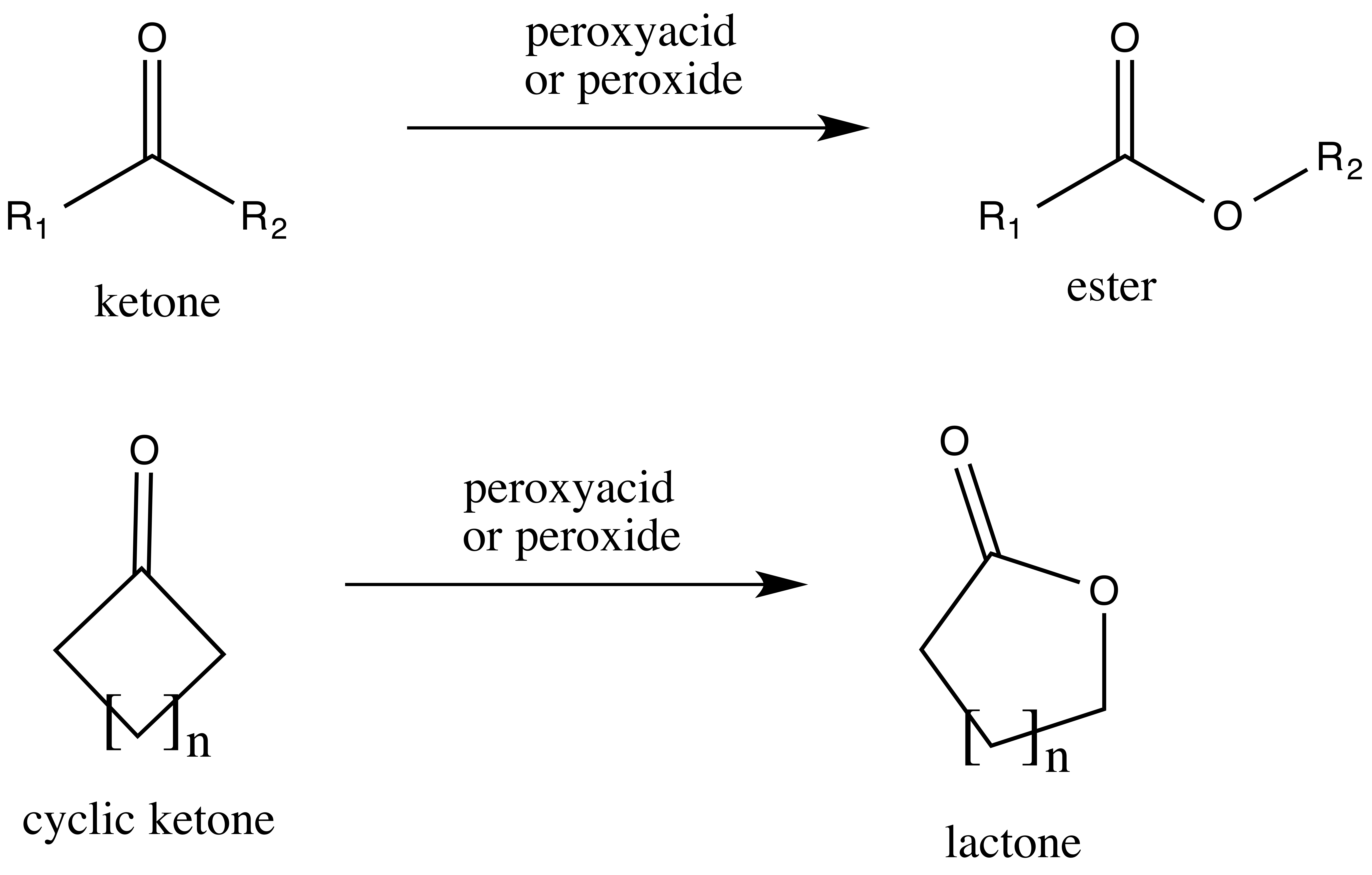
Baeyer–Villiger Oxidation
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899. Reaction mechanism In the first step of the reaction mechanism, the peroxyacid protonates the oxygen of the carbonyl group. This makes the carbonyl group more susceptible to be attacked by the peroxyacid. Next, the peroxyacid attacks the carbon of the carbonyl group forming what is known as the Criegee intermediate. Through a concerted mechanism, one of the substituents on the ketone group migrates to the oxygen of the peroxide group while a carboxylic acid leaves. This migration step is thought to be the rate determining step. Finally, deprotonation of the oxocarbenium ion produces the ester. The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and seconda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
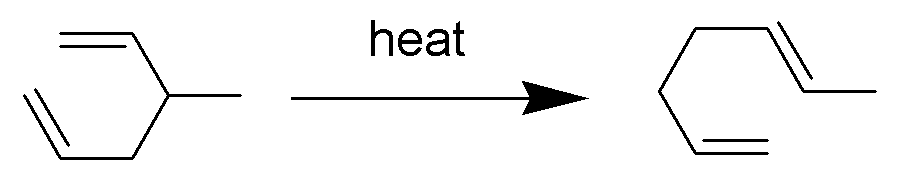
Cope Rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene. The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family. Mechanism The Cope rearrangement is the prototypical example of a concerted sigmatropic rearrangement. It is classified as a ,3sigmatropic rearrangement with the Woodward–Hoffmann symbol π2s+σ2s+π2s">sub>π2s+σ2s+π2sand is therefore thermally allowed. It is sometimes useful to think of it as going through a transition state energetically and structurally equivalent to a diradical, although the diradical is not usually a true intermediate (potential energy minimum). The chair transition state illustrated here is preferred in open-chain systems (as shown by the Doering-Roth experiments). However, conformationally co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beckmann Rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams. The Beckmann rearrangement is often catalyzed by acid; however, other reagents have been known to promote the rearrangement. These include tosyl chloride, thionyl chloride, phosphorus pentachloride, phosphorus pentoxide, triethylamine, sodium hydroxide, trimethylsilyl iodide among others. The Beckmann fragmentation is another reaction that often competes with the rearrangement, though careful selection of promoting reagent and solvent conditions can favor the formation of one over the other, sometimes giving almost exclusively one product. The rearrangement occurs stereospecifically for ketoximes and N-chloro/N-fluoro imines, with the migrating group being anti-periplanar to the leaving gro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heck Reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a substituted alkene. It is named after Tsutomu Mizoroki and Richard F. Heck. Heck was awarded the 2010 Nobel Prize in Chemistry, which he shared with Ei-ichi Negishi and Akira Suzuki, for the discovery and development of this reaction. This reaction was the first example of a carbon-carbon bond-forming reaction that followed a Pd(0)/Pd(II) catalytic cycle, the same catalytic cycle that is seen in other Pd(0)-catalyzed cross-coupling reactions. The Heck reaction is a way to substitute alkenes. History The original reaction by Tsutomu Mizoroki (1971) describes the coupling between iodobenzene and styrene in methanol to form stilbene at 120 °C (autoclave) with potassium acetate base and palladium chloride catalysis. This work was a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |