Diels–Alder Reaction on:
[Wikipedia]
[Google]
[Amazon]
In
 The "prevailing opinion" Carey, Part A., p. 839 is that most Diels–Alder reactions proceed through a concerted mechanism; the issue, however, has been thoroughly contested. Despite the fact that the vast majority of Diels–Alder reactions exhibit stereospecific, syn addition of the two components, a diradical intermediate has been postulated (and supported with computational evidence) on the grounds that the observed stereospecificity does not rule out a two-step addition involving an intermediate that collapses to product faster than it can rotate to allow for inversion of stereochemistry.
There is a notable rate enhancement when certain Diels–Alder reactions are carried out in polar organic solvents such as
The "prevailing opinion" Carey, Part A., p. 839 is that most Diels–Alder reactions proceed through a concerted mechanism; the issue, however, has been thoroughly contested. Despite the fact that the vast majority of Diels–Alder reactions exhibit stereospecific, syn addition of the two components, a diradical intermediate has been postulated (and supported with computational evidence) on the grounds that the observed stereospecificity does not rule out a two-step addition involving an intermediate that collapses to product faster than it can rotate to allow for inversion of stereochemistry.
There is a notable rate enhancement when certain Diels–Alder reactions are carried out in polar organic solvents such as
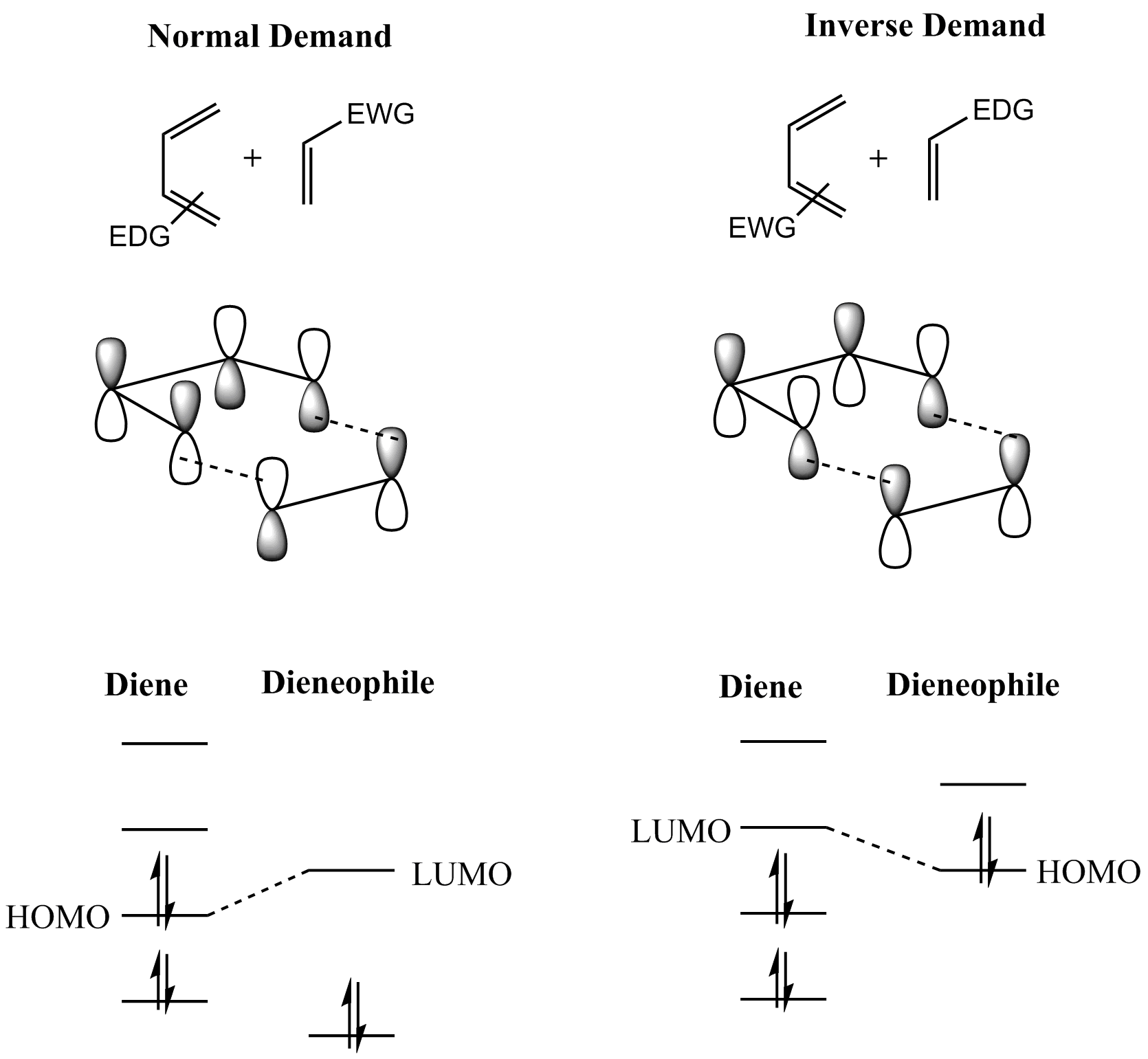
 In general, the regioselectivity found for both normal and inverse electron-demand Diels–Alder reaction follows the ortho-para rule, so named, because the cyclohexene product bears substituents in positions that are analogous to the ''ortho'' and ''para'' positions of disubstituted arenes. For example, in a normal-demand scenario, a diene bearing an electron-donating group (EDG) at C1 has its largest HOMO coefficient at C4, while the dienophile with an electron withdrawing group (EWG) at C1 has the largest LUMO coefficient at C2. Pairing these two coefficients gives the "ortho" product as seen in case 1 in the figure below. A diene substituted at C2 as in case 2 below has the largest HOMO coefficient at C1, giving rise to the "para" product. Similar analyses for the corresponding inverse-demand scenarios gives rise to the analogous products as seen in cases 3 and 4. Examining the canonical mesomeric forms above, it is easy to verify that these results are in accord with expectations based on consideration of electron density and polarization.
In general, the regioselectivity found for both normal and inverse electron-demand Diels–Alder reaction follows the ortho-para rule, so named, because the cyclohexene product bears substituents in positions that are analogous to the ''ortho'' and ''para'' positions of disubstituted arenes. For example, in a normal-demand scenario, a diene bearing an electron-donating group (EDG) at C1 has its largest HOMO coefficient at C4, while the dienophile with an electron withdrawing group (EWG) at C1 has the largest LUMO coefficient at C2. Pairing these two coefficients gives the "ortho" product as seen in case 1 in the figure below. A diene substituted at C2 as in case 2 below has the largest HOMO coefficient at C1, giving rise to the "para" product. Similar analyses for the corresponding inverse-demand scenarios gives rise to the analogous products as seen in cases 3 and 4. Examining the canonical mesomeric forms above, it is easy to verify that these results are in accord with expectations based on consideration of electron density and polarization.
 In general, with respect to the energetically most well-matched HOMO-LUMO pair, maximizing the interaction energy by forming bonds between centers with the largest frontier orbital coefficients allows the prediction of the main regioisomer that will result from a given diene-dienophile combination. In a more sophisticated treatment, three types of substituents (Z ''withdrawing'': HOMO and LUMO lowering (CF3, NO2, CN, C(O)CH3), X ''donating'': HOMO and LUMO raising (Me, OMe, NMe2), C ''conjugating'': HOMO raising and LUMO lowering (Ph, vinyl)) are considered, resulting in a total of 18 possible combinations. The maximization of orbital interaction correctly predicts the product in all cases for which experimental data is available. For instance, in uncommon combinations involving X groups on both diene and dienophile, a 1,3-substitution pattern may be favored, an outcome not accounted for by a simplistic resonance structure argument. However, cases where the resonance argument and the matching of largest orbital coefficients disagree are rare.
In general, with respect to the energetically most well-matched HOMO-LUMO pair, maximizing the interaction energy by forming bonds between centers with the largest frontier orbital coefficients allows the prediction of the main regioisomer that will result from a given diene-dienophile combination. In a more sophisticated treatment, three types of substituents (Z ''withdrawing'': HOMO and LUMO lowering (CF3, NO2, CN, C(O)CH3), X ''donating'': HOMO and LUMO raising (Me, OMe, NMe2), C ''conjugating'': HOMO raising and LUMO lowering (Ph, vinyl)) are considered, resulting in a total of 18 possible combinations. The maximization of orbital interaction correctly predicts the product in all cases for which experimental data is available. For instance, in uncommon combinations involving X groups on both diene and dienophile, a 1,3-substitution pattern may be favored, an outcome not accounted for by a simplistic resonance structure argument. However, cases where the resonance argument and the matching of largest orbital coefficients disagree are rare.

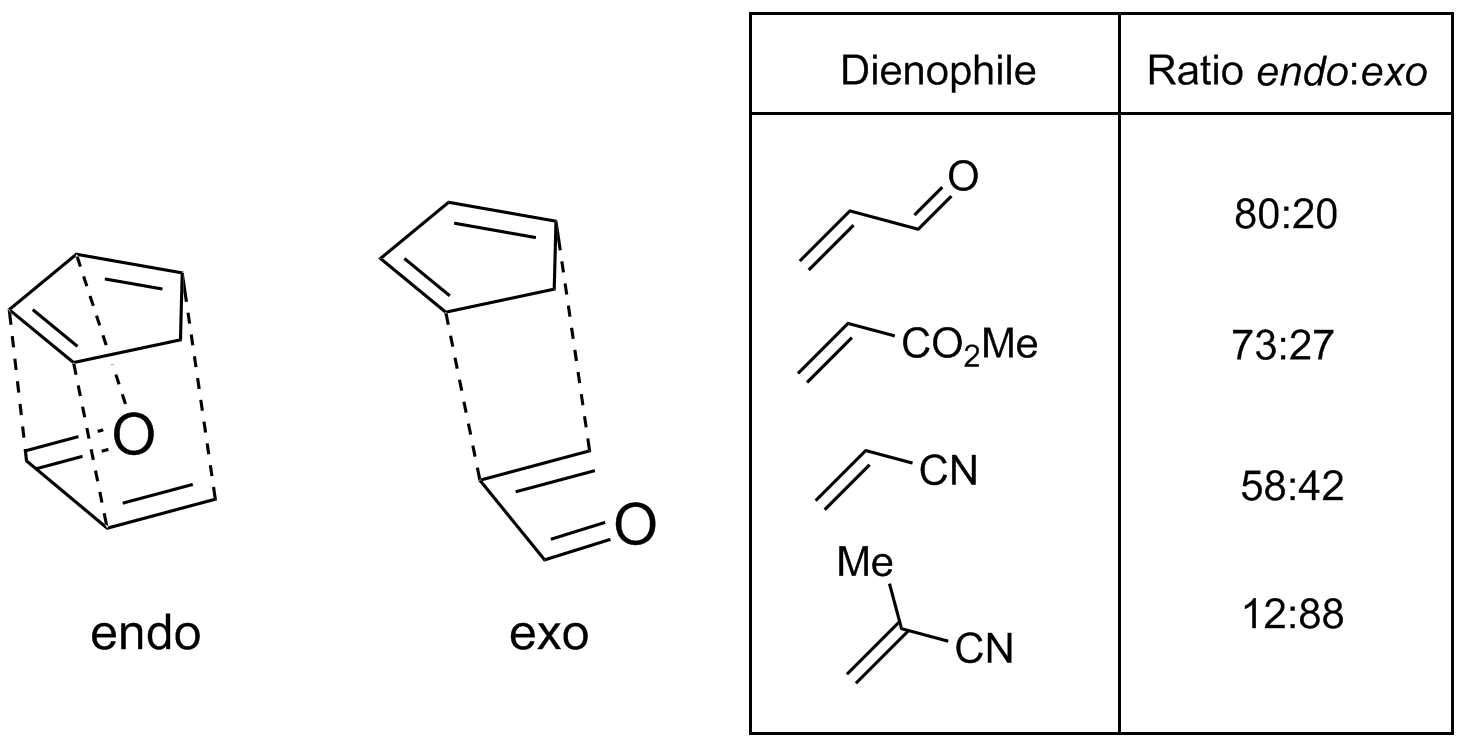 Diels–Alder reactions in which adjacent stereocenters are generated at the two ends of the newly formed single bonds imply two different possible stereochemical outcomes. This is a stereoselective situation based on the relative orientation of the two separate components when they react with each other. In the context of the Diels–Alder reaction, the transition state in which the most significant substituent (an electron-withdrawing and/or conjugating group) on the dienophile is oriented towards the diene π system and slips under it as the reaction takes place is known as the ''endo'' transition state. In the alternative ''exo'' transition state, it is oriented away from it. (There is a more general usage of the terms ''endo'' and ''exo'' in stereochemical nomenclature.)
In cases where the dienophile has a single electron-withdrawing / conjugating substituent, or two electron-withdrawing / conjugating substituents ''cis'' to each other, the outcome can often be predicted. In these "normal demand" Diels–Alder scenarios, the ''endo'' transition state is typically preferred, despite often being more sterically congested. This preference is known as the Alder endo rule. As originally stated by Alder, the transition state that is preferred is the one with a "maximum accumulation of double bonds." ''Endo'' selectivity is typically higher for rigid dienophiles such as
Diels–Alder reactions in which adjacent stereocenters are generated at the two ends of the newly formed single bonds imply two different possible stereochemical outcomes. This is a stereoselective situation based on the relative orientation of the two separate components when they react with each other. In the context of the Diels–Alder reaction, the transition state in which the most significant substituent (an electron-withdrawing and/or conjugating group) on the dienophile is oriented towards the diene π system and slips under it as the reaction takes place is known as the ''endo'' transition state. In the alternative ''exo'' transition state, it is oriented away from it. (There is a more general usage of the terms ''endo'' and ''exo'' in stereochemical nomenclature.)
In cases where the dienophile has a single electron-withdrawing / conjugating substituent, or two electron-withdrawing / conjugating substituents ''cis'' to each other, the outcome can often be predicted. In these "normal demand" Diels–Alder scenarios, the ''endo'' transition state is typically preferred, despite often being more sterically congested. This preference is known as the Alder endo rule. As originally stated by Alder, the transition state that is preferred is the one with a "maximum accumulation of double bonds." ''Endo'' selectivity is typically higher for rigid dienophiles such as  The most widely accepted explanation for the origin of this effect is a favorable interaction between the π systems of the dienophile and the diene, an interaction described as a ''secondary orbital effect'', though dipolar and van der Waals attractions may play a part as well, and solvent can sometimes make a substantial difference in selectivity. The secondary orbital overlap explanation was first proposed by Woodward and Hoffmann. In this explanation, the orbitals associated with the group in conjugation with the dienophile double-bond overlap with the interior orbitals of the diene, a situation that is possible only for the ''endo'' transition state. Although the original explanation only invoked the orbital on the atom α to the dienophile double bond, Salem and Houk have subsequently proposed that orbitals on the α and β carbons both participate when molecular geometry allows.
The most widely accepted explanation for the origin of this effect is a favorable interaction between the π systems of the dienophile and the diene, an interaction described as a ''secondary orbital effect'', though dipolar and van der Waals attractions may play a part as well, and solvent can sometimes make a substantial difference in selectivity. The secondary orbital overlap explanation was first proposed by Woodward and Hoffmann. In this explanation, the orbitals associated with the group in conjugation with the dienophile double-bond overlap with the interior orbitals of the diene, a situation that is possible only for the ''endo'' transition state. Although the original explanation only invoked the orbital on the atom α to the dienophile double bond, Salem and Houk have subsequently proposed that orbitals on the α and β carbons both participate when molecular geometry allows.
 Often, as with highly substituted dienes, very bulky dienophiles, or
Often, as with highly substituted dienes, very bulky dienophiles, or
 Unstable (and thus highly reactive) dienes can be synthetically useful, e.g. ''o''- quinodimethanes can be generated in situ. In contrast, stable dienes, such as
Unstable (and thus highly reactive) dienes can be synthetically useful, e.g. ''o''- quinodimethanes can be generated in situ. In contrast, stable dienes, such as
 The retro-Diels–Alder reaction is used in the industrial production of
The retro-Diels–Alder reaction is used in the industrial production of 
 The Diels-Alder reaction was the culmination of several intertwined research threads, some near misses, and ultimately, the insightful recognition of a general principle by Otto Diels and Kurt Alder. Their seminal work, detailed in a series of 28 articles published in the '' Justus Liebigs Annalen der Chemie'' and ''
The Diels-Alder reaction was the culmination of several intertwined research threads, some near misses, and ultimately, the insightful recognition of a general principle by Otto Diels and Kurt Alder. Their seminal work, detailed in a series of 28 articles published in the '' Justus Liebigs Annalen der Chemie'' and ''
 Diels–Alder reactions were used in the original synthesis of
Diels–Alder reactions were used in the original synthesis of  A Diels–Alder reaction is a key step in the synthesis of (-)-furaquinocin C.
A Diels–Alder reaction is a key step in the synthesis of (-)-furaquinocin C.
 (+)-Sterpurene can be prepared by asymmetric D-A reaction that featured a remarkable intramolecular Diels–Alder reaction of an
(+)-Sterpurene can be prepared by asymmetric D-A reaction that featured a remarkable intramolecular Diels–Alder reaction of an 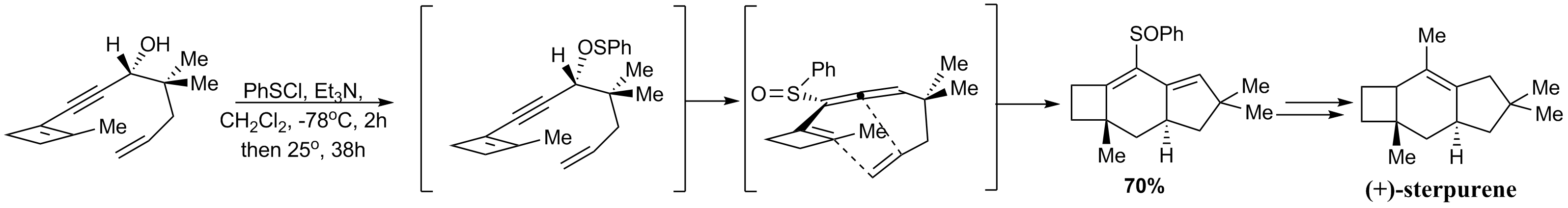 The tetracyclic core of the antibiotic (-)-tetracycline was prepared with a Diels–Alder reaction. Thermally initiated, conrotatory opening of the benzocyclobutene generated the ''o''-quinodimethane, which reacted intermolecularly to give the tetracycline skeleton. The dienophile's free hydroxyl group is integral to the success of the reaction, as hydroxyl-protected variants did not react under several different reaction conditions.
The tetracyclic core of the antibiotic (-)-tetracycline was prepared with a Diels–Alder reaction. Thermally initiated, conrotatory opening of the benzocyclobutene generated the ''o''-quinodimethane, which reacted intermolecularly to give the tetracycline skeleton. The dienophile's free hydroxyl group is integral to the success of the reaction, as hydroxyl-protected variants did not react under several different reaction conditions.
 Takemura et al. synthesized
Takemura et al. synthesized
English Translation of Diels and Alder's seminal 1928 German article that won them the Nobel prize. English title: 'Syntheses of the hydroaromatic series'; German title "Synthesen in der hydroaromatischen Reihe". {{DEFAULTSORT:Diels-Alder reaction Cycloadditions Carbon-carbon bond forming reactions Ring forming reactions German inventions 1928 in science 1928 in Germany Name reactions
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, the Diels–Alder reaction is a chemical reaction between a conjugated diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
and a substituted alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
, commonly termed the dienophile, to form a substituted cyclohexene
Cyclohexene is a hydrocarbon with the formula . It is a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an chemical intermediate, intermediate in the commercial synthesis of nylon.
Prod ...
derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally allowed +2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
with Woodward–Hoffmann symbol sub>π4s + π2s It was first described by Otto Diels and Kurt Alder
Kurt Alder (; 10 July 1902 – 20 June 1958) was a German chemist and Nobel laureate.
Biography
Alder was born in the industrial area of Königshütte, Silesia (modern day Chorzów, Upper Silesia, Poland), where he received his early schoo ...
in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry
The Nobel Prize in Chemistry () is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outst ...
in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
s, such as carbonyls and imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of Δ''H''° and Δ''S''° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels–Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.
Mechanism
The reaction is an example of a concerted pericyclic reaction. Carey, Part B., pp. 474–526 It is believed to occur via a single, cyclic transition state, with no intermediates generated during the course of the reaction. As such, the Diels–Alder reaction is governed by orbital symmetry considerations: it is classified as a sub>π4s + π2scycloaddition, indicating that it proceeds through the suprafacial/suprafacial interaction of a 4π electron system (the diene structure) with a 2π electron system (the dienophile structure), an interaction that leads to a transition state without an additional orbital symmetry-imposed energetic barrier and allows the Diels–Alder reaction to take place with relative ease. Carey, Part A., pp. 836–50 A consideration of the reactants' frontier molecular orbitals (FMO) makes plain why this is so. (The same conclusion can be drawn from an orbital correlation diagram or a Dewar-Zimmerman analysis.) For the more common "normal" electron demand Diels–Alder reaction, the more important of the two HOMO/LUMO interactions is that between the electron-rich diene's ''ψ''2 as the highest occupied molecular orbital (HOMO) with the electron-deficient dienophile's π* as the lowest unoccupied molecular orbital (LUMO). However, the HOMO–LUMO energy gap is close enough that the roles can be reversed by switching electronic effects of the substituents on the two components. In an inverse (reverse) electron-demand Diels–Alder reaction, electron-withdrawing substituents on the diene lower the energy of its empty ''ψ''3 orbital and electron-donating substituents on the dienophile raise the energy of its filled π orbital sufficiently that the interaction between these two orbitals becomes the most energetically significant stabilizing orbital interaction. Regardless of which situation pertains, the HOMO and LUMO of the components are in phase and a bonding interaction results as can be seen in the diagram below. Since the reactants are in their ground state, the reaction is initiated thermally and does not require activation by light. The "prevailing opinion" Carey, Part A., p. 839 is that most Diels–Alder reactions proceed through a concerted mechanism; the issue, however, has been thoroughly contested. Despite the fact that the vast majority of Diels–Alder reactions exhibit stereospecific, syn addition of the two components, a diradical intermediate has been postulated (and supported with computational evidence) on the grounds that the observed stereospecificity does not rule out a two-step addition involving an intermediate that collapses to product faster than it can rotate to allow for inversion of stereochemistry.
There is a notable rate enhancement when certain Diels–Alder reactions are carried out in polar organic solvents such as
The "prevailing opinion" Carey, Part A., p. 839 is that most Diels–Alder reactions proceed through a concerted mechanism; the issue, however, has been thoroughly contested. Despite the fact that the vast majority of Diels–Alder reactions exhibit stereospecific, syn addition of the two components, a diradical intermediate has been postulated (and supported with computational evidence) on the grounds that the observed stereospecificity does not rule out a two-step addition involving an intermediate that collapses to product faster than it can rotate to allow for inversion of stereochemistry.
There is a notable rate enhancement when certain Diels–Alder reactions are carried out in polar organic solvents such as dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liqui ...
and ethylene glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
, and even in water. The reaction of cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
This colorless liquid has a strong and unpleasant odor. At room temperature, ...
and butenone for example is 700 times faster in water relative to 2,2,4-trimethylpentane
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale ...
as solvent. Several explanations for this effect have been proposed, such as an increase in effective concentration due to hydrophobic packing or hydrogen-bond stabilization of the transition state.
The geometry of the diene and dienophile components each propagate into stereochemical details of the product. For intermolecular reactions especially, the preferred positional and stereochemical relationship of substituents of the two components compared to each other are controlled by electronic effects. However, for intramolecular Diels–Alder cycloaddition reactions, the conformational stability of the structure of the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
can be an overwhelming influence.
Regioselectivity
Frontier molecular orbital theory has also been used to explain the regioselectivity patterns observed in Diels–Alder reactions of substituted systems. Calculation of the energy and orbital coefficients of the components' frontier orbitals provides a picture that is in good accord with the more straightforward analysis of the substituents' resonance effects, as illustrated below. In general, the regioselectivity found for both normal and inverse electron-demand Diels–Alder reaction follows the ortho-para rule, so named, because the cyclohexene product bears substituents in positions that are analogous to the ''ortho'' and ''para'' positions of disubstituted arenes. For example, in a normal-demand scenario, a diene bearing an electron-donating group (EDG) at C1 has its largest HOMO coefficient at C4, while the dienophile with an electron withdrawing group (EWG) at C1 has the largest LUMO coefficient at C2. Pairing these two coefficients gives the "ortho" product as seen in case 1 in the figure below. A diene substituted at C2 as in case 2 below has the largest HOMO coefficient at C1, giving rise to the "para" product. Similar analyses for the corresponding inverse-demand scenarios gives rise to the analogous products as seen in cases 3 and 4. Examining the canonical mesomeric forms above, it is easy to verify that these results are in accord with expectations based on consideration of electron density and polarization.
In general, the regioselectivity found for both normal and inverse electron-demand Diels–Alder reaction follows the ortho-para rule, so named, because the cyclohexene product bears substituents in positions that are analogous to the ''ortho'' and ''para'' positions of disubstituted arenes. For example, in a normal-demand scenario, a diene bearing an electron-donating group (EDG) at C1 has its largest HOMO coefficient at C4, while the dienophile with an electron withdrawing group (EWG) at C1 has the largest LUMO coefficient at C2. Pairing these two coefficients gives the "ortho" product as seen in case 1 in the figure below. A diene substituted at C2 as in case 2 below has the largest HOMO coefficient at C1, giving rise to the "para" product. Similar analyses for the corresponding inverse-demand scenarios gives rise to the analogous products as seen in cases 3 and 4. Examining the canonical mesomeric forms above, it is easy to verify that these results are in accord with expectations based on consideration of electron density and polarization.
 In general, with respect to the energetically most well-matched HOMO-LUMO pair, maximizing the interaction energy by forming bonds between centers with the largest frontier orbital coefficients allows the prediction of the main regioisomer that will result from a given diene-dienophile combination. In a more sophisticated treatment, three types of substituents (Z ''withdrawing'': HOMO and LUMO lowering (CF3, NO2, CN, C(O)CH3), X ''donating'': HOMO and LUMO raising (Me, OMe, NMe2), C ''conjugating'': HOMO raising and LUMO lowering (Ph, vinyl)) are considered, resulting in a total of 18 possible combinations. The maximization of orbital interaction correctly predicts the product in all cases for which experimental data is available. For instance, in uncommon combinations involving X groups on both diene and dienophile, a 1,3-substitution pattern may be favored, an outcome not accounted for by a simplistic resonance structure argument. However, cases where the resonance argument and the matching of largest orbital coefficients disagree are rare.
In general, with respect to the energetically most well-matched HOMO-LUMO pair, maximizing the interaction energy by forming bonds between centers with the largest frontier orbital coefficients allows the prediction of the main regioisomer that will result from a given diene-dienophile combination. In a more sophisticated treatment, three types of substituents (Z ''withdrawing'': HOMO and LUMO lowering (CF3, NO2, CN, C(O)CH3), X ''donating'': HOMO and LUMO raising (Me, OMe, NMe2), C ''conjugating'': HOMO raising and LUMO lowering (Ph, vinyl)) are considered, resulting in a total of 18 possible combinations. The maximization of orbital interaction correctly predicts the product in all cases for which experimental data is available. For instance, in uncommon combinations involving X groups on both diene and dienophile, a 1,3-substitution pattern may be favored, an outcome not accounted for by a simplistic resonance structure argument. However, cases where the resonance argument and the matching of largest orbital coefficients disagree are rare.
Stereospecificity and stereoselectivity
Diels–Alder reactions, as concerted cycloadditions, are stereospecific. Stereochemical information of the diene and the dienophile are retained in the product, as a ''syn'' addition with respect to each component. For example, substituents in a ''cis'' (''trans'', resp.) relationship on the double bond of the dienophile give rise to substituents that are ''cis'' (''trans'', resp.) on those same carbons with respect to the cyclohexene ring. Likewise, ''cis'',''cis''- and ''trans'',''trans''-disubstituted dienes give ''cis'' substituents at these carbons of the product whereas ''cis'',''trans''-disubstituted dienes give ''trans'' substituents:
 Diels–Alder reactions in which adjacent stereocenters are generated at the two ends of the newly formed single bonds imply two different possible stereochemical outcomes. This is a stereoselective situation based on the relative orientation of the two separate components when they react with each other. In the context of the Diels–Alder reaction, the transition state in which the most significant substituent (an electron-withdrawing and/or conjugating group) on the dienophile is oriented towards the diene π system and slips under it as the reaction takes place is known as the ''endo'' transition state. In the alternative ''exo'' transition state, it is oriented away from it. (There is a more general usage of the terms ''endo'' and ''exo'' in stereochemical nomenclature.)
In cases where the dienophile has a single electron-withdrawing / conjugating substituent, or two electron-withdrawing / conjugating substituents ''cis'' to each other, the outcome can often be predicted. In these "normal demand" Diels–Alder scenarios, the ''endo'' transition state is typically preferred, despite often being more sterically congested. This preference is known as the Alder endo rule. As originally stated by Alder, the transition state that is preferred is the one with a "maximum accumulation of double bonds." ''Endo'' selectivity is typically higher for rigid dienophiles such as
Diels–Alder reactions in which adjacent stereocenters are generated at the two ends of the newly formed single bonds imply two different possible stereochemical outcomes. This is a stereoselective situation based on the relative orientation of the two separate components when they react with each other. In the context of the Diels–Alder reaction, the transition state in which the most significant substituent (an electron-withdrawing and/or conjugating group) on the dienophile is oriented towards the diene π system and slips under it as the reaction takes place is known as the ''endo'' transition state. In the alternative ''exo'' transition state, it is oriented away from it. (There is a more general usage of the terms ''endo'' and ''exo'' in stereochemical nomenclature.)
In cases where the dienophile has a single electron-withdrawing / conjugating substituent, or two electron-withdrawing / conjugating substituents ''cis'' to each other, the outcome can often be predicted. In these "normal demand" Diels–Alder scenarios, the ''endo'' transition state is typically preferred, despite often being more sterically congested. This preference is known as the Alder endo rule. As originally stated by Alder, the transition state that is preferred is the one with a "maximum accumulation of double bonds." ''Endo'' selectivity is typically higher for rigid dienophiles such as maleic anhydride
Maleic anhydride is an organic compound with the formula . It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.
Str ...
and benzoquinone; for others, such as acrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain ...
s and crotonates, selectivity is not very pronounced.
 The most widely accepted explanation for the origin of this effect is a favorable interaction between the π systems of the dienophile and the diene, an interaction described as a ''secondary orbital effect'', though dipolar and van der Waals attractions may play a part as well, and solvent can sometimes make a substantial difference in selectivity. The secondary orbital overlap explanation was first proposed by Woodward and Hoffmann. In this explanation, the orbitals associated with the group in conjugation with the dienophile double-bond overlap with the interior orbitals of the diene, a situation that is possible only for the ''endo'' transition state. Although the original explanation only invoked the orbital on the atom α to the dienophile double bond, Salem and Houk have subsequently proposed that orbitals on the α and β carbons both participate when molecular geometry allows.
The most widely accepted explanation for the origin of this effect is a favorable interaction between the π systems of the dienophile and the diene, an interaction described as a ''secondary orbital effect'', though dipolar and van der Waals attractions may play a part as well, and solvent can sometimes make a substantial difference in selectivity. The secondary orbital overlap explanation was first proposed by Woodward and Hoffmann. In this explanation, the orbitals associated with the group in conjugation with the dienophile double-bond overlap with the interior orbitals of the diene, a situation that is possible only for the ''endo'' transition state. Although the original explanation only invoked the orbital on the atom α to the dienophile double bond, Salem and Houk have subsequently proposed that orbitals on the α and β carbons both participate when molecular geometry allows.
 Often, as with highly substituted dienes, very bulky dienophiles, or
Often, as with highly substituted dienes, very bulky dienophiles, or reversible reaction
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously.
: \mathit aA + \mathit bB \mathit cC + \mathit dD
A and B can react to form C and D or, in the ...
s (as in the case of furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as f ...
as diene), steric effects can override the normal ''endo'' selectivity in favor of the ''exo'' isomer.
The diene
Thediene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
component of the Diels–Alder reaction can be either open-chain or cyclic, and it can host many different types of substituents. It must, however, be able to exist in the s-''cis'' conformation, since this is the only conformer that can participate in the reaction. Though butadienes are typically more stable in the s-''trans'' conformation, for most cases energy difference is small (~2–5 kcal/mol).
A bulky substituent at the C2 or C3 position can increase reaction rate by destabilizing the s-''trans'' conformation and forcing the diene into the reactive s-''cis'' conformation. 2-''tert''-butyl-buta-1,3-diene, for example, is 27 times more reactive than simple butadiene. Conversely, a diene having bulky substituents at both C2 and C3 is less reactive because the steric interactions between the substituents destabilize the s-''cis'' conformation.
Dienes with bulky terminal substituents (C1 and C4) decrease the rate of reaction, presumably by impeding the approach of the diene and dienophile.
An especially reactive diene is 1-methoxy-3-trimethylsiloxy-buta-1,3-diene, otherwise known as Danishefsky's diene. It has particular synthetic utility as means of furnishing α,β–unsaturated cyclohexenone systems by elimination of the 1-methoxy substituent after deprotection of the enol silyl ether. Other synthetically useful derivatives of Danishefsky's diene include 1,3-alkoxy-1-trimethylsiloxy-1,3-butadienes (Brassard dienes) and 1-dialkylamino-3-trimethylsiloxy-1,3-butadienes (Rawal dienes). The increased reactivity of these and similar dienes is a result of synergistic contributions from donor groups at C1 and C3, raising the HOMO significantly above that of a comparable monosubstituted diene.
 Unstable (and thus highly reactive) dienes can be synthetically useful, e.g. ''o''- quinodimethanes can be generated in situ. In contrast, stable dienes, such as
Unstable (and thus highly reactive) dienes can be synthetically useful, e.g. ''o''- quinodimethanes can be generated in situ. In contrast, stable dienes, such as naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
, require forcing conditions and/or highly reactive dienophiles, such as ''N''-phenylmaleimide. Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes, as a scintil ...
, being less aromatic (and therefore more reactive for Diels–Alder syntheses) in its central ring can form a 9,10 adduct with maleic anhydride
Maleic anhydride is an organic compound with the formula . It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.
Str ...
at 80 °C and even with acetylene
Acetylene (Chemical nomenclature, systematic name: ethyne) is a chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is u ...
, a weak dienophile, at 250 °C.
The dienophile
In a normal demand Diels–Alder reaction, the dienophile has an electron-withdrawing group in conjugation with the alkene; in an inverse-demand scenario, the dienophile is conjugated with an electron-donating group. Dienophiles can be chosen to contain a "masked functionality". The dienophile undergoes Diels–Alder reaction with a diene introducing such a functionality onto the product molecule. A series of reactions then follow to transform the functionality into a desirable group. The end product cannot be made in a single DA step because equivalent dienophile is either unreactive or inaccessible. An example of such approach is the use of α-chloroacrylonitrile (CH2=CClCN). When reacted with a diene, this dienophile will introduce α-chloronitrile functionality onto the product molecule. This is a "masked functionality" which can be then hydrolyzed to form aketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
. α-Chloroacrylonitrile dienophile is an equivalent of ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
dienophile (CH2=C=O), which would produce same product in one DA step. The problem is that ketene itself cannot be used in Diels–Alder reactions because it reacts with dienes in unwanted manner (by +2cycloaddition), and therefore "masked functionality" approach has to be used. Other such functionalities are phosphonium
In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. The ...
substituents (yielding exocyclic double bonds after Wittig reaction), various sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
and sulfonyl functionalities (both are acetylene equivalents), and nitro group
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nit ...
s (ketene equivalents).
Variants on the classical Diels–Alder reaction
Hetero-Diels–Alder
Diels–Alder reactions involving at least oneheteroatom
In chemistry, a heteroatom () is, strictly, any atom that is not carbon or hydrogen.
Organic chemistry
In practice, the term is mainly used more specifically to indicate that non-carbon atoms have replaced carbon in the backbone of the molecular ...
are also known and are collectively called hetero-Diels–Alder reactions. Carbonyl group
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes ...
s, for example, can successfully react with dienes to yield dihydropyran In organic chemistry, dihydropyran refers to two heterocyclic compounds with the formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of ...
rings, a reaction known as the oxo-Diels–Alder reaction, and imines
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
can be used, either as the dienophile or at various sites in the diene, to form various ''N''-heterocyclic compounds through the aza-Diels–Alder reaction. Nitroso compounds (R-N=O) can react with dienes to form oxazines. Chlorosulfonyl isocyanate can be utilized as a dienophile to prepare Vince lactam.
Lewis acid activation
Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s, such as zinc chloride
Zinc chloride is an Inorganic chemistry, inorganic chemical compound with the chemical formula, formula ZnCl2·''n''H2O, with ''n'' ranging from 0 to 4.5, forming water of hydration, hydrates. Zinc chloride, anhydrous and its hydrates, are colo ...
, boron trifluoride
Boron trifluoride is the inorganic compound with the formula . This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bonding
The g ...
, tin tetrachloride, or aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col ...
, can catalyze Diels–Alder reactions by binding to the dienophile. Traditionally, the enhanced Diels-Alder reactivity is ascribed to the ability of the Lewis acid to lower the LUMO of the activated dienophile, which results in a smaller normal electron demand HOMO-LUMO orbital energy gap and hence more stabilizing orbital interactions.
Recent studies, however, have shown that this rationale behind Lewis acid-catalyzed Diels–Alder reactions is incorrect. It is found that Lewis acids accelerate the Diels–Alder reaction by reducing the destabilizing steric Pauli repulsion between the interacting diene and dienophile and not by lowering the energy of the dienophile's LUMO and consequently, enhancing the normal electron demand orbital interaction. The Lewis acid binds via a donor-acceptor interaction to the dienophile and via that mechanism polarizes occupied orbital density away from the reactive C=C double bond of the dienophile towards the Lewis acid. This reduced occupied orbital density on C=C double bond of the dienophile will, in turn, engage in a less repulsive closed-shell-closed-shell orbital interaction with the incoming diene, reducing the destabilizing steric Pauli repulsion and hence lowers the Diels–Alder reaction barrier. In addition, the Lewis acid catalyst also increases the asynchronicity of the Diels–Alder reaction, making the occupied π-orbital located on the C=C double bond of the dienophile asymmetric. As a result, this enhanced asynchronicity leads to an extra reduction of the destabilizing steric Pauli repulsion as well as a diminishing pressure on the reactants to deform, in other words, it reduced the destabilizing activation strain (also known as distortion energy). This working catalytic mechanism is known as ''Pauli-lowering catalysis'', which is operative in a variety of organic reactions.
The original rationale behind Lewis acid-catalyzed Diels–Alder reactions is incorrect, because besides lowering the energy of the dienophile's LUMO, the Lewis acid also lowers the energy of the HOMO of the dienophile and hence increases the inverse electron demand LUMO-HOMO orbital energy gap. Thus, indeed Lewis acid catalysts strengthen the normal electron demand orbital interaction by lowering the LUMO of the dienophile, but, they simultaneously weaken the inverse electron demand orbital interaction by also lowering the energy of the dienophile's HOMO. These two counteracting phenomena effectively cancel each other, resulting in nearly unchanged orbital interactions when compared to the corresponding uncatalyzed Diels–Alder reactions and making this not the active mechanism behind Lewis acid-catalyzed Diels–Alder reactions.
Asymmetric Diels–Alder
Many methods have been developed for influencing the stereoselectivity of the Diels–Alder reaction, such as the use of chiral auxiliaries, catalysis by chiral Lewis acids, and small organic molecule catalysts. Evans' oxazolidinones, oxazaborolidines, ''bis''-oxazoline–copperchelate
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
s, imidazoline catalysis, and many other methodologies exist for effecting diastereo- and enantioselective Diels–Alder reactions.
Hexadehydro Diels–Alder
In the hexadehydro Diels–Alder reaction,alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s and diynes are used instead of alkenes and dienes, forming an unstable benzyne intermediate which can then be trapped to form an aromatic product. This reaction allows the formation of heavily functionalized aromatic rings in a single step.
Applications and natural occurrence
cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
This colorless liquid has a strong and unpleasant odor. At room temperature, ...
. Cyclopentadiene is a precursor to various norbornenes, which are common monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s. The Diels–Alder reaction is also employed in the production of vitamin B6
Vitamin B6 is one of the B vitamins, and is an essential nutrient for humans. The term essential nutrient refers to a group of six chemically similar compounds, i.e., "vitamers", which can be interconverted in biological systems. Its active f ...
.
History
Berichte der deutschen chemischen Gesellschaft
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with ' ...
'' from 1928 to 1937, established the reaction's wide applicability and its importance in constructing six-membered rings. The first 19 articles were authored by Diels and Alder, while the later articles were authored by Diels and various other coauthors. However, the history of the reaction extends further back, revealing a fascinating narrative of discoveries missed and opportunities overlooked.
Several chemists, working independently in the late 19th and early 20th centuries, encountered reactions that, in retrospect, involved the Diels-Alder process but remained unrecognized as such.
* Theodor Zincke
Ernst Carl Theodor Zincke (19 May 1843 – 17 March 1928) was a German chemist and the academic adviser of Otto Hahn.
Life
Theodor Zincke was born in Uelzen on 19 May 1843. He became a pharmacist and graduated in Göttingen with his Staatsexamen ...
performed a series of experiments between 1892 and 1912 involving tetrachlorocyclopentadienone, a highly reactive diene analogue.
* In 1910, Sergey Lebedev systematically investigated thermal polymerization of three conjugated dienes (butadiene, isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers ar ...
and dimethylbutadiene), a process now recognized as a Diels-Alder self-reaction, providing a detailed analysis of the dimerization products and recognizing the importance of the conjugated system in the process. Five years earlier, Carl Harries
Carl Dietrich Harries (5 August 1866 – 3 November 1923) was a German chemist born in Luckenwalde, Brandenburg, Prussia. He received his doctorate in 1892. In 1900, he married Hertha von Siemens, daughter of the electrical genius Werner von Si ...
studied the degradation of natural rubber, leading him to propose a cyclic structure for the polymer.
* Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
's work with ketenes published in 1912 covered both +2cycloadditions, where one molecule of a ketene reacted with an unsaturated compound to form a four-membered ring, and, importantly, +2cycloadditions. In the latter case, two molecules of ketene combined with one molecule of an unsaturated compound (such as a quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with ...
) to yield a six-membered ring. While not a classic Diels-Alder reaction in the typical sense of a conjugated diene and a separate dienophile, Staudinger's observation of this +2process, forming a six-membered ring, foreshadowed the later work of Diels and Alder. However, his focus remained primarily on the more common +2ketene cycloaddition.
* Hans von Euler-Chelpin and K. O. Josephson, investigating isoprene and butadiene reactions in 1920, both observed products consistent with Diels-Alder cycloadditions, but didn't go on to research it further.
* Perhaps the most striking near miss came from Walter Albrecht in early 1900s. Working in Johannes Thiele Johannes Thiele may refer to:
*Johannes Thiele (zoologist)
*Johannes Thiele (chemist)
{{hndis, Thiele, Johannes ...
's laboratory, Albrecht investigated the reaction of cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.
This colorless liquid has a strong and unpleasant odor. At room temperature, ...
with para-benzoquinone. His 1902 doctoral dissertation clearly describes the formation of the Diels-Alder adduct, even providing (incorrect) structural assignments. However, influenced by Thiele's focus on conjugation and partial valence, Albrecht in his 1906 publication interpreted the reaction as a 1,4-addition followed by a 1,2-addition, completely overlooking the cycloaddition aspect.
While these observations hinted at the possibility of a broader class of cycloaddition reactions, they remained isolated incidents, their significance not fully appreciated at the time, with none of the researchers even trying to generalize their findings.
It fell to Diels and Alder to synthesize these disparate threads into a coherent whole. Unlike the earlier researchers, they recognized the generality and predictability of the diene and dienophile combining to form a cyclic structure. Through their systematic investigations, exploring various combinations of dienes and dienophiles, they firmly established the "diene synthesis" as a powerful new synthetic method. Their meticulous work not only demonstrated the reaction's scope and versatility but also laid the groundwork for future theoretical developments, including the Woodward-Hoffmann rules, which would provide a deeper understanding of pericyclic reactions, including the Diels-Alder.
Applications in total synthesis
The Diels–Alder reaction was one step in an early preparation of the steroidscortisone
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally-occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug. Cortisol is converted by the action of the enzyme corticosteroid 11-beta-dehydrogenase ...
and cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
. The reaction involved the addition of butadiene to a quinone.
 Diels–Alder reactions were used in the original synthesis of
Diels–Alder reactions were used in the original synthesis of prostaglandin
Prostaglandins (PG) are a group of physiology, physiologically active lipid compounds called eicosanoids that have diverse hormone-like effects in animals. Prostaglandins have been found in almost every Tissue (biology), tissue in humans and ot ...
s F2α and E2. The Diels–Alder reaction establishes the relative stereochemistry of three contiguous stereocenters on the prostaglandin cyclopentane core. Activation by Lewis acidic cupric tetrafluoroborate was required.
A Diels–Alder reaction was used in the synthesis of disodium prephenate, a biosynthetic precursor of the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the chemical formula, formula . It can be viewed as a benzyl group substituent, substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of ...
and tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is ...
.
A synthesis of reserpine
Reserpine is a drug that is used for the treatment of hypertension, high blood pressure, usually in combination with a thiazide diuretic or vasodilator. Large clinical trials have shown that combined treatment with reserpine plus a thiazide diur ...
uses a Diels–Alder reaction to set the ''cis''-decalin framework of the D and E rings.
In another synthesis of reserpine, the ''cis''-fused D and E rings was formed by a Diels–Alder reaction. Intramolecular Diels–Alder of the pyranone below with subsequent extrusion of carbon dioxide via a retro +2afforded the bicyclic lactam
A lactam is a Cyclic compound, cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek_alphabet#Letters, Greek prefixes in alpha ...
. Epoxidation from the less hindered α-face, followed by epoxide opening at the less hindered C18 afforded the desired stereochemistry at these positions, while the ''cis''-fusion was achieved with hydrogenation, again proceeding primarily from the less hindered face.
A pyranone was similarly used as the dienophile in the total synthesis of taxol. The intermolecular reaction of the hydroxy-pyrone and α,β–unsaturated ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
shown below suffered from poor yield and regioselectivity; however, when directed by phenylboronic acid the desired adduct could be obtained in 61% yield after cleavage of the boronate with neopentyl glycol. The stereospecificity of the Diels–Alder reaction in this instance allowed for the definition of four stereocenters that were carried on to the final product.
 A Diels–Alder reaction is a key step in the synthesis of (-)-furaquinocin C.
A Diels–Alder reaction is a key step in the synthesis of (-)-furaquinocin C.
Tabersonine
Tabersonine is a terpene indole alkaloid found in the medicinal plant '' Catharanthus roseus'' and also in the genus Voacanga (both taxa belonging to the alkaloid-rich family Apocynaceae). Tabersonine is hydroxylated at the 16 position by the en ...
was prepared by a Diels–Alder reaction to establish ''cis'' relative stereochemistry of the alkaloid core. Conversion of the ''cis''-aldehyde to its corresponding alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
by Wittig olefination and subsequent ring-closing metathesis
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various Saturated and unsaturated compounds, unsaturated rings via the intramolecular olefin metathesis, metathesis of two term ...
with a Schrock catalyst gave the second ring of the alkaloid core. The diene in this instance is notable as an example of a 1-amino-3-siloxybutadiene, otherwise known as a Rawal diene.
 (+)-Sterpurene can be prepared by asymmetric D-A reaction that featured a remarkable intramolecular Diels–Alder reaction of an
(+)-Sterpurene can be prepared by asymmetric D-A reaction that featured a remarkable intramolecular Diels–Alder reaction of an allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
. The ,3sigmatropic rearrangement of the thiophenyl group to give the sulfoxide as below proceeded enantiospecifically due to the predefined stereochemistry of the propargylic alcohol. In this way, the single allene isomer formed could direct the Diels–Alder reaction to occur on only one face of the generated 'diene'.
 The tetracyclic core of the antibiotic (-)-tetracycline was prepared with a Diels–Alder reaction. Thermally initiated, conrotatory opening of the benzocyclobutene generated the ''o''-quinodimethane, which reacted intermolecularly to give the tetracycline skeleton. The dienophile's free hydroxyl group is integral to the success of the reaction, as hydroxyl-protected variants did not react under several different reaction conditions.
The tetracyclic core of the antibiotic (-)-tetracycline was prepared with a Diels–Alder reaction. Thermally initiated, conrotatory opening of the benzocyclobutene generated the ''o''-quinodimethane, which reacted intermolecularly to give the tetracycline skeleton. The dienophile's free hydroxyl group is integral to the success of the reaction, as hydroxyl-protected variants did not react under several different reaction conditions.
 Takemura et al. synthesized
Takemura et al. synthesized cantharidin
Cantharidin is an odorless, colorless fatty substance of the terpenoid class, which is secreted by many species of blister beetles. Its main current use in pharmacology is treating molluscum contagiosum and warts topically. It is a burn agent ...
in 1980 by Diels–Alder reaction, utilizing high pressure.
Synthetic applications of the Diels–Alder reaction have been reviewed extensively.
See also
* Bradsher cycloaddition * Wagner-Jauregg reaction * Aza-Diels–Alder reactionReferences
Bibliography
*External links
English Translation of Diels and Alder's seminal 1928 German article that won them the Nobel prize. English title: 'Syntheses of the hydroaromatic series'; German title "Synthesen in der hydroaromatischen Reihe". {{DEFAULTSORT:Diels-Alder reaction Cycloadditions Carbon-carbon bond forming reactions Ring forming reactions German inventions 1928 in science 1928 in Germany Name reactions