|
R-404a
This is a list of refrigerants, sorted by their ASHRAE-designated numbers, commonly known as R numbers. Many modern refrigerants are human-made halogenated gases, especially fluorinated gases and chlorinated gases, that are frequently referred to as Freon (a registered trademark of Chemours). Freons are responsible for the formation of the ozone hole. The Vienna Convention for the Protection of the Ozone Layer and the Montreal Protocol are international agreements that oblige signatory countries to limit the emission of ozone-depleting gases. The Kigali Amendment to the Montreal Protocol furthermore obliges signatory countries to limit the emission of gases with high global warming potential. Numbering scheme According to ASHRAE standard 34, the R-number of a chemical refrigerant is assigned systematically according to its molecular structure and has between two and four digits. If there are carbon-carbon multiple bonds, there are four digits in all: the number of these bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ASHRAE
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE ) is an American professional association seeking to advance heating, ventilation, air conditioning and refrigeration (HVAC&R) systems design and construction. ASHRAE has over 50,000 members in more than 130 countries worldwide. ASHRAE's members comprise building services engineers, architects, mechanical contractors, building owners, equipment manufacturers' employees, and others concerned with the design and construction of HVAC&R systems in buildings. The society funds research projects, offers continuing education programs, and develops and publishes technical standards to improve building services engineering, energy efficiency, indoor air quality, and sustainable development. History ASHRAE was founded in 1894 at a meeting of engineers in New York City, formerly headquartered at 345 East 47th Street (the United Engineering Center), and has held an annual meeting since 1895. Until ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorodifluoromethane
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or . It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in Developed country, developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene. Production and current applications Worldwide production of R-22 in 2008 was about 800Gg per year, up from about 450Gg per year in 1998, with most production in developing countries. R-22 use is being phased out in developing countries, where it is largely used for air conditioning applications. R-22 is prepared from chloroform: :HCCl3 + 2 HF → HCF2Cl + 2 HCl An important application ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature
IUPAC nomenclature is a set of recommendations for naming chemical compounds and for describing chemistry and biochemistry in general. The International Union of Pure and Applied Chemistry (IUPAC) is the international authority on chemical nomenclature and terminology. History of the Standardisation of Nomenclature In 1787, Louis-Bernard Guyton de Morveau published his nomenclature recommendations in collaboration with fellow French chemists Berthollet, de Fourcroy and Lavoisier. This work however covered only what are now called inorganic compounds. With the expansion of organic chemistry in the 19th century, and a greater understanding of the structure of organic compounds, the need for a more global standardised nomenclature became more prominent. Following a series of meetings, the first of which was established in 1860 by August Kekulé, the Geneva Nomenclature of 1892 was created. Another entity called the International Association of Chemical Societies (IACS) exis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type And Flammability
Type may refer to: Science and technology Computing * Typing, producing text via a keyboard, typewriter, etc. * Data type, collection of values used for computations. * File type * TYPE (DOS command), a command to display contents of a file. * Type (Unix), a command in POSIX shells that gives information about commands. * Type safety, the extent to which a programming language discourages or prevents type errors. * Type system, defines a programming language's response to data types. Mathematics * Type (model theory) * Type theory, basis for the study of type systems * Arity or type, the number of operands a function takes * Type, any proposition or set in the intuitionistic type theory * Type, of an entire function ** Exponential type Biology * Type (biology), which fixes a scientific name to a taxon * Dog type, categorization by use or function of domestic dogs Lettering * Type is a design concept for lettering used in typography which helped bring about modern textual printi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
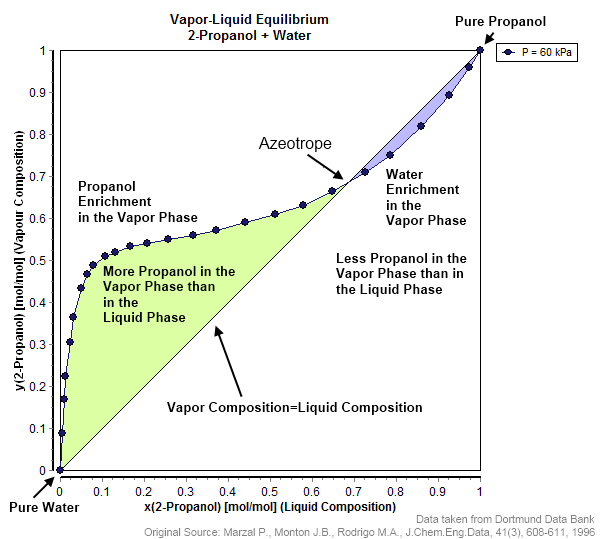
Azeotropic
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Knowing an azeotrope's behavior is important for distillation. Each azeotrope has a characteristic boiling point. The boiling point of an azeotrope is either less than the boiling point temperatures of any of its constituents (a positive azeotrope), or greater than the boiling point of any of its constituents (a negative azeotrope). For both positive and negative azeotropes, it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. For technical applications, the pressure-temperature-composition behavior of a mixture is the most important, but other important ther ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeotropic
A zeotropic mixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram. Zeotropic mixtures have different characteristics in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis–trans Isomerism
''Cis''–''trans'' isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules. The prefixes "''cis''" and "''trans''" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, ''cis'' indicates that the functional groups (substituents) are on the same side of some plane, while ''trans'' conveys that they are on opposing (transverse) sides. ''Cis''–''trans'' isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. ''Cis'' and ''trans'' isomers occur both in organic molecules and in inorganic coordination complexes. ''Cis'' and ''trans'' descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "''syn''" and "''anti''" are used. According to IUPAC, "geome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E–Z Notation
''E''–''Z'' configuration, or the ''E''–''Z'' convention, is the IUPAC preferred method of describing the absolute stereochemistry of double bonds in organic chemistry. It is an extension of ''cis''–''trans'' isomer notation (which only describes ''relative stereochemistry'') that can be used to describe double bonds having two, three or four substituents. E and Z notation are only used when a compound doesn't have two identical substituents. Following the Cahn–Ingold–Prelog priority rules (CIP rules), each substituent on a double bond is assigned a priority, then positions of the higher of the two substituents on each carbon are compared to each other. If the two groups of higher priority are on opposite sides of the double bond (''trans'' to each other), the bond is assigned the configuration ''E'' (from ''entgegen'', , the German word for "opposite"). If the two groups of higher priority are on the same side of the double bond (''cis'' to each other), the bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the existence or possibility of isomers. Isomers do not necessarily share similar chemical property, chemical or physical property, physical properties. Two main forms of isomerism are structural isomerism, structural (or constitutional) isomerism, in which ''chemical bond, bonds'' between the atoms differ; and stereoisomerism (or spatial isomerism), in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different Isotopologue, isotopologues. The depth of analy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |