|
Programmed Cell Death Protein 1
Programmed cell death protein 1 (PD-1), (CD279 cluster of differentiation 279). PD-1 is a protein encoded in humans by the ''PDCD1'' gene. PD-1 is a cell surface receptor on T cells and B cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. This prevents autoimmune diseases, but it can also prevent the immune system from killing cancer cells. PD-1 is an immune checkpoint and guards against autoimmunity through two mechanisms. First, it promotes apoptosis (programmed cell death) of antigen-specific T-cells in lymph nodes. Second, it reduces apoptosis in regulatory T cells (anti-inflammatory, suppressive T cells). PD-1 inhibitors, a new class of drugs that block PD-1, activate the immune system to attack tumors and are used to treat certain types of cancer. PD-1 is a cell surface receptor that belongs to the immunoglobulin sup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Of Differentiation
The cluster of differentiation (also known as cluster of designation or classification determinant and often abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules providing targets for immunophenotyping of cells. In terms of physiology, CD molecules can act in numerous ways, often acting as receptors or ligands important to the cell. A signal cascade is usually initiated, altering the behavior of the cell (see cell signaling). Some CD proteins do not play a role in cell signaling, but have other functions, such as cell adhesion. CD for humans is numbered up to 371 (). Nomenclature The CD nomenclature was proposed and established in the 1st International Workshop and Conference on Human Leukocyte Differentiation Antigens (HLDA), held in Paris in 1982. This system was intended for the classification of the many monoclonal antibodies (mAbs) generated by different laboratories around the world against epitopes on the surface mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PD-L1
Programmed death-ligand 1 (PD-L1) also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) is a protein that in humans is encoded by the ''CD274'' gene. Programmed death-ligand 1 (PD-L1) is a 40kDa type 1 transmembrane protein that has been speculated to play a major role in suppressing the adaptive arm of immune systems during particular events such as pregnancy, tissue allografts, autoimmune disease and other disease states such as hepatitis. Normally the adaptive immune system reacts to antigens that are associated with immune system activation by exogenous or endogenous danger signals. In turn, clonal expansion of antigen-specific CD8+ T cells and/or CD4+ helper cells is propagated. The binding of PD-L1 to the inhibitory checkpoint molecule PD-1 transmits an inhibitory signal based on interaction with phosphatases ( SHP-1 or SHP-2) via Immunoreceptor Tyrosine-Based Switch Motif (ITSM). This reduces the proliferation of antigen-specific T-cells in l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTPN6
Tyrosine-protein phosphatase non-receptor type 6, also known as Src homology region 2 domain-containing phosphatase-1 (SHP-1), is an enzyme that in humans is encoded by the ''PTPN6'' gene. Function The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. N-terminal part of this PTP contains two tandem Src homolog ( SH2) domains, which act as protein phospho-tyrosine binding domains, and mediate the interaction of this PTP with its substrates. This PTP is expressed primarily in hematopoietic cells, and functions as an important regulator of multiple signaling pathways in hematopoietic cells. This PTP has been shown to interact with, and dephosphorylate a wide spectrum of phospho-proteins involved in hematopoietic cell signaling, (e.g., the LYN-CD22-SHP-1 pathway). Multip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T-cell Receptor
The T-cell receptor (TCR) is a protein complex, located on the surface of T cells (also called T lymphocytes). They are responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is biologically degenerate (that is, many TCRs recognize the same antigen peptide, and many antigen peptides are recognized by the same TCR). The TCR is composed of two different protein chains (that is, it is a hetero dimer). In humans, in 95% of T cells the TCR consists of an alpha (α) chain and a beta (β) chain (encoded by '' TRA'' and ''TRB'', respectively), whereas in 5% of T cells the TCR consists of gamma and delta (γ/δ) chains (encoded by '' TRG'' and '' TRD'', respectively). This ratio changes during ontogeny and in diseased states (such as leukemia). It also differs between species. Orthologues of the 4 loci have been mapped in various speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoreceptor Tyrosine-based Inhibitory Motif
An immunoreceptor tyrosine-based inhibitory motif (ITIM), is a conserved sequence of amino acids that is found intracellularly in the cytoplasmic domains of many inhibitory receptors of the non-catalytic tyrosine-phosphorylated receptor family found on immune cells. These immune cells include T cells, B cells, NK cells, dendritic cells, macrophages and mast cells. ITIMs have similar structures of S/I/V/LxYxxI/V/L, where x is any amino acid, Y is a tyrosine residue that can be phosphorylated, S is the amino acid serine, I is the amino acid isoleucine, and V is the amino acid valine. ITIMs recruit SH2 domain-containing phosphatases, which inhibit cellular activation. ITIM-containing receptors often serve to target immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors, resulting in an innate inhibition mechanism within cells. ITIM bearing receptors have important role in regulation of immune system allowing negative regulation at different levels of the immune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols: : This equation can be written in several ways that are nearly equivalent that describe the behaviors of various protonated states of ATP, ADP, and the phosphorylated product. As is clear from the equation, a phosphate group per se is not transferred, but a phosphoryl group (PO3-). Phosphoryl is an electrophile. This process and its inverse, dephosphorylation, are common in biology. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. During respiration Phosphorylation is essential to the processes of both anaerobic and aerobic respiration, which involve the production of adenosine triphosphate (ATP), the "high-energy" exc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-pass membrane proteins, or as multipass membrane proteins. Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Superfamily
The immunoglobulin superfamily (IgSF) is a large protein superfamily of cell surface and soluble proteins that are involved in the recognition, binding, or adhesion processes of cells. Molecules are categorized as members of this superfamily based on shared structural features with immunoglobulins (also known as antibodies); they all possess a domain known as an immunoglobulin domain or fold. Members of the IgSF include cell surface antigen receptors, co-receptors and co-stimulatory molecules of the immune system, molecules involved in antigen presentation to lymphocytes, cell adhesion molecules, certain cytokine receptors and intracellular muscle proteins. They are commonly associated with roles in the immune system. Otherwise, the sperm-specific protein IZUMO1, a member of the immunoglobulin superfamily, has also been identified as the only sperm membrane protein essential for sperm-egg fusion. Immunoglobulin domains Proteins of the IgSF possess a structural domain kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CTLA-4
Cytotoxic T-lymphocyte associated protein 4, (CTLA-4) also known as CD152 ( cluster of differentiation 152), is a protein receptor that functions as an immune checkpoint and downregulates immune responses. CTLA-4 is constitutively expressed in regulatory T cells but only upregulated in conventional T cells after activation – a phenomenon which is particularly notable in cancers. It acts as an "off" switch when bound to CD80 or CD86 on the surface of antigen-presenting cells. It is encoded by the gene ''CTLA4'' in humans. The CTLA-4 protein is encoded by the ''Ctla-4'' gene in mice. History CTLA-4 was first identified in 1991 as a second receptor for the T cell costimulation ligand B7. In November 1995, the labs of Tak Wah Mak and Arlene Sharpe independently published their findings on the discovery of the function of CTLA-4 as a negative regulator of T-cell activation, by knocking out the gene in mice. Previous studies from several labs had used methods which could ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD28
CD28 (Cluster of Differentiation 28) is a protein expressed on T cells that provides essential co-stimulation, co-stimulatory signals required for T cell activation and survival. When T cells are stimulated through CD28 in conjunction with the T-cell receptor (T cell receptor, TCR), it enhances the production of various interleukins, particularly interleukin 6, IL-6. CD28 serves as a receptor for CD80 (B7.1) and CD86 (B7.2), proteins found on antigen-presenting cells (APCs). CD28 is the only B7 (protein), B7 receptor consistently expressed on naive T cells. In the absence of CD28:B7 interaction, a naive T cell's TCR engagement with an Major histocompatibility complex, MHC:antigen complex leads to anergy. CD28 is also expressed on bone marrow stromal cells, plasma cells, neutrophils, and eosinophils, although its function in these cells is not fully understood. Typically, CD28 is expressed on about 50% of CD8, CD8+ T cells and more than 80% of CD4, CD4+ T cells in humans. However, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
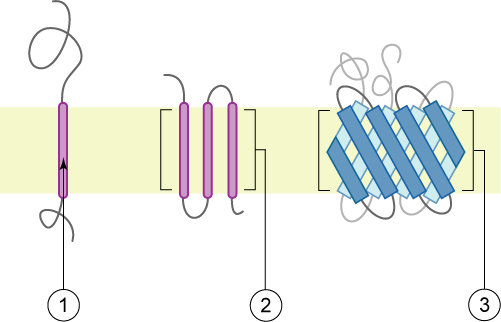
Type I Membrane Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to the network of interactions between different proteins in cells, including interactions via transmembrane alpha helices. They usually include one or several water-soluble domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. More than 2300 single-pass membrane proteins were identified in the human genome. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |