|
Phosphines
Organophosphines are organophosphorus compounds with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphines (''n'' = 2), tertiary phosphines (''n'' = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3). Annette Schier and Hubert Schmidbaur"P-Donor Ligands" in Encyclopedia of Inorganic Chemistry 2006, Wiley-VCH, Weinheim. 1° vs 2° vs 3° phosphines Organophophines are classified according to the number of organic substituents. Primary phosphines Primary (1°) phosphines, with the formula RPH2, in principle are derived by alkylation of phosphine. Some simple alkyl derivatives such as methylphosphine (CH3PH2) can be prepared by alkylation of phosphine in the presence of base: : (M = Li, Na, K) A more common syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophosphination
Hydrophosphination is the insertion of a double bond into a phosphorus-hydrogen bond. Often the hydrophosphination makes phosphorus-carbon bonds by addition of P-H bonds to carbon-carbon multiple bonds, but the reaction is probably most useful in reactions of phosphine with formaldehyde, a form of hydroxymethylation. Like other hydrofunctionalizations, the rate and regiochemistry of the insertion reaction is influenced by the catalyst. Catalysts take many forms, but most prevalent are bases and free-radical initiators. Most hydrophosphinations involve reactions of phosphine (PH3). Hydroxyalkylation Although most of this article concerns addition of P-H bonds to alkenes, an important variant is the hydrophosphination of formaldehyde. Tetrakis(hydroxymethyl)phosphonium chloride (THPC) is prepared as follows from phosphine: :PH3 + 4 H2C=O + HCl → [P(CH2OH)4]Cl It is a white water-soluble salt with applications as a precursor to fire-retardant materials and as a microbiocide in co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX (nerve agent), VX nerve agents. Phosphorus, like nitrogen, is in pnictogen, group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt a v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
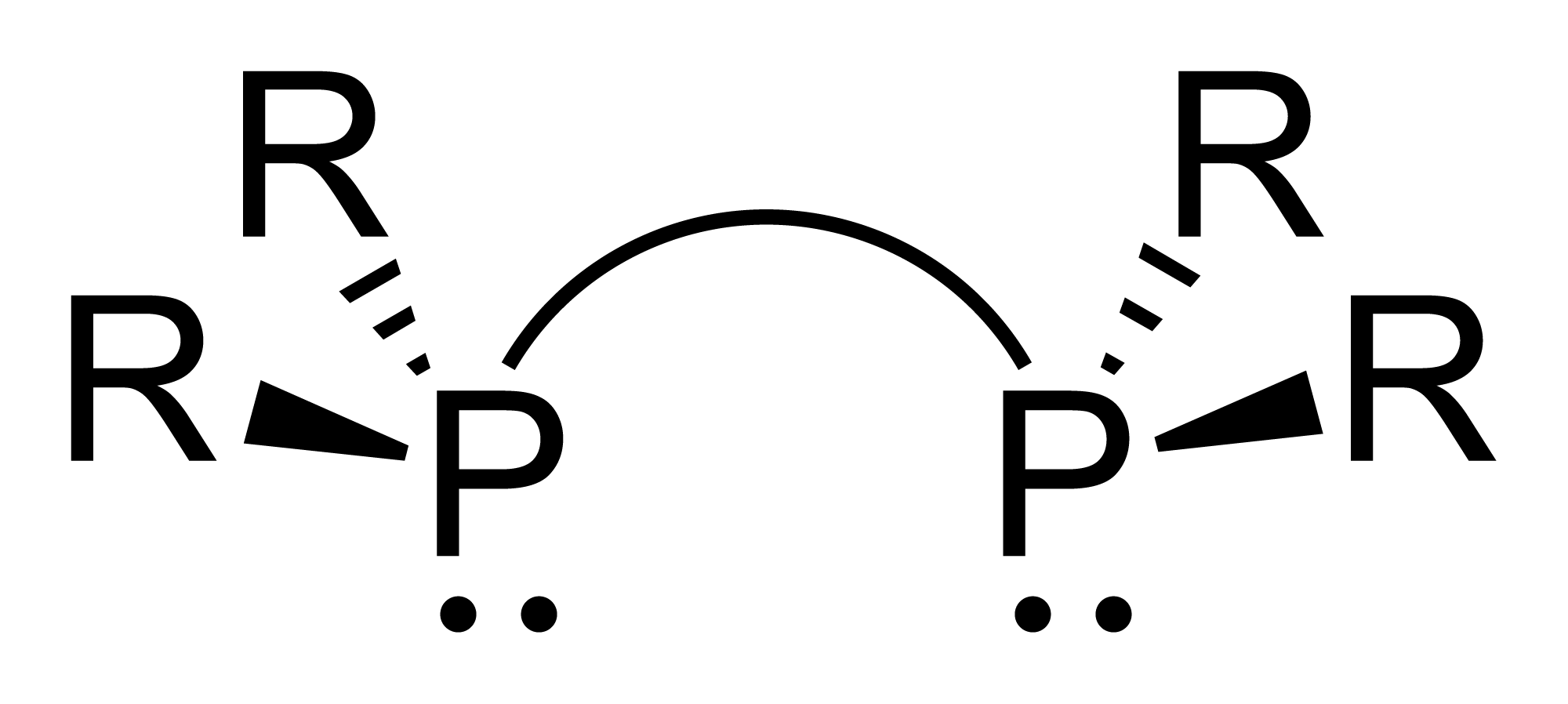
Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligand, phosphine ligands in inorganic chemistry, inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backbone, and are usually chelate, chelating. A wide variety of diphosphines have been synthesized with different linkers and R-groups. Alteration of the linker and R-groups alters the electronic and steric properties of the ligands which can result in different coordination geometries and catalytic behavior in Homogeneous catalysis, homogeneous catalysts. Synthesis image:IPr2PCl.png, 220px, Chlorodiisopropylphosphine is a popular building block for the preparation of diphosphines. From phosphide building blocks Many widely used diphosphine ligands have the general formula Ar2P(CH2)nPAr2. These compounds can be prepared from the reaction of X(CH2)nX (X=halogen) and MPPh2 (M = alkali metal): :Cl(CH2)nCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorophenylphosphine
Dichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of organophosphines. Dichlorophenylphosphine is commercially available. It may be prepared by an electrophilic substitution of benzene by phosphorus trichloride, catalyzed by aluminium chloride Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col .... However, aluminum chloride often induces diarylation; a cleaner catalyst for monoarylation is stannic chloride. The compound is an intermediate for the synthesis of other chemicals for instance dimethylphenylphosphine: :C6H5PCl2 + 2 CH3MgI → C6H5P(CH3)2 + 2 MgICl Many tertiary phosphines can be prepared by this route. In the McCormack reaction dichlorophenylphosphine adds dienes to give the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylphosphine
Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts. Synthesis Diphenylphosphine can be prepared from triphenylphosphine by reduction to lithium diphenylphosphide, which can be protonated to give the title compound: :PPh3 + 2 Li → LiPPh2 + LiPh :LiPPh2 + H2O → Ph2PH + LiOH Uses and reactions In the laboratory, diphenylphosphine is a common intermediate. It can be deprotonated to give diphenylphosphide derivatives: :Ph2PH + nBuLi → Ph2PLi + nBuH The preparation of phosphine ligands, Wittig-Horner reagents, and phosphonium salts are commonly accomplished by alkylating diphenylphosphine. The hydrogen atom connected to phosphorus undergoes Michael-like addition to activated alkenes, providing products with which to produce phosphine ligands such as 1,2-bis(diphenylphosphino)ethane (Ph2PCH2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the thre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine () is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Diphenylphosphide
Lithium diphenylphosphide contains lithium and the organophosphorus anion with the formula . It is a red, air-sensitive solid that is used in the preparation of diphenylphosphino compounds. Synthesis and reactions The lithium, sodium, and potassium salts are prepared by reduction of chlorodiphenylphosphine, triphenylphosphine, or tetraphenyldiphosphine with alkali metals (M): : : : They can also be obtained by deprotonation of diphenylphosphine. With water, the salts convert to diphenylphosphine: : With halocarbon Halocarbon compounds are chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, or ...s, the salts react to give tertiary phosphines: : When treated with metal halides, lithium diphenylphosphide gives transition metal phosphido complexes. Structure and physical properties Although treated as salt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylphosphine
Phenylphosphine is an organophosphorus compound with the chemical formula C6H5PH2. It is the phosphorus analog of aniline. Like other primary phosphines, phenylphosphine has an intense penetrating odor and is highly oxidizable. It is mainly used as a precursor to other organophosphorus compounds. It can function as a ligand in coordination chemistry. Synthesis Phenylphosphine can be produced by reducing dichlorophenylphosphine with lithium aluminum hydride in ether: :LiAlH4 + 2C6H5PCl2 → 2C6H5PH2 + Li+ + Al3+ + 4Cl− This reaction is performed under a nitrogen atmosphere to prevent side reactions involving oxygen. Reactions Oxidation of phenylphosphine with air affords the oxide. :C6H5PH2 + O2 → C6H5P(OH)2 Bis(2-cyanoethylphenyl)phosphine, which is of interest as a synthetic intermediate, can be made from phenylphosphine by base-catalyzed allyl In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attach ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P-Chiral Phosphine
''P''-Chiral phosphines are organophosphorus compounds of the formula PRR′R″, where R, R′, R″ = H, alkyl, aryl, etc. They are a subset of chiral phosphines, a broader class of compounds where the stereogenic center can reside at sites other than phosphorus. P-chirality exploits the high barrier for inversion of phosphines, which ensures that enantiomers of PRR'R" do not racemize readily. The inversion barrier is relatively insensitive to substituents for triorganophosphines. By contrast, most amines of the type NRR′R″ undergo rapid pyramidal inversion. Research themes Most chiral phosphines are C2-symmetric diphosphines. Famous examples are DIPAMP and BINAP. These chelating ligands support catalysts used in asymmetric hydrogenation and related reactions. DIPAMP is prepared by coupling the ''P''-chiral methylphenylanisylphosphine. ''P''-Chiral phosphines are of particular interest in asymmetric catalysis. ''P''-Chiral phosphines have been investigated for two m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylphosphine
Methylphosphine is the simplest organophosphorus compound with the formula CH3PH2, often written MePH2. It is a malodorous gas that condenses to a colorless liquid. It can be produced by methylation of phosphanide salts: :KPH2 + MeI → MePH2 + KI Reactions The compound exhibits the properties characteristic of a primary phosphine, i.e., a compound of the type RPH2. It can be oxidized to methylphosphonous acid: :MePH2 + O2 → MeP(H)O2H It protonates to give a phosphonium In chemistry, the term phosphonium (more obscurely: phosphinium) describes polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, organyl or halogen group). These cations have tetrahedral structures. The ... ion: :MePH2 + H+ → MePH3+ With strong bases, it can be deprotonated to give methylphosphanide derivatives: :MePH2 + KOH → K ePH + H2O References {{Reflist Phosphines Foul-smelling chemicals Methyl compounds Organic compounds wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |