|
Phenyl Azide
Phenyl azide is an organic compound with the formula C6H5N3. It is one of the prototypical organic azides. It is a pale yellow oily liquid with a pungent odor. The structure consists of a linear azide substituent bound to a phenyl group. The C−N=N angle is approximately 116°. It was discovered in 1864 by Peter Griess by the reaction of ammonia and phenyldiazonium. Preparation Phenyl azide is prepared by the diazotization of phenylhydrazine with nitrous acid: :C6H5NHNH2 + HNO2 → C6H5N3 + 2 H2O Aryl iodides bearing electron-withdrawing substituents undergo metathesis with sodium azide in the presence of Cu(I), sodium ascorbate, and N,N'-dimethylethane-1,2-diamine (DMEDA): :RC6H4I + NaN3 → RC6H4N3 + NaI It can also be prepared by condensation of benzenediazonium salt with toluenesulfonamide, followed by hydrolysis. Chemical reactions Phenyl azide cycloadds to alkenes and especially alkynes, particularly those bearing electronegative substituents. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Dimethylethylenediamine
N,N'-Dimethylethylenediamine (DMEDA) is the organic compound with the formula (CHNH)CH. It is a colorless liquid with a fishy odor. It features two secondary amine functional groups. Regarding its name, N and N' indicate that the methyl groups are attached to different nitrogen atoms. Reactions DMEDA is used as a chelating diamine for the preparation of metal complexes, some of which function as homogeneous catalysts. The compound is used as a precursor to imidazolidines by condensation with ketones or with aldehydes: : DMEDA complexes of copper(I) halides are used to catalyze C-N coupling reactions. See also * 1,1-Dimethylethylenediamine * Dimethylaminopropylamine Dimethylaminopropylamine (DMAPA) is a diamine used in the preparation of some surfactants, such as cocamidopropyl betaine which is an ingredient in many personal care products including soaps, shampoos, and cosmetics. BASF, a major producer, clai ... References {{DEFAULTSORT:Dimethylethylenediamine, 1, 2- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl Compounds
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen atom, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with and is represented by the symbol Ph (archaically, Φ), or Ø. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Footnotes
In publishing, a note is a brief text in which the author comments on the subject and themes of the book and names supporting citations. In the editorial production of books and documents, typographically, a note is usually several lines of text at the bottom of the page, at the end of a chapter, at the end of a volume, or a house-style typographic usage throughout the text. Notes are usually identified with superscript numbers or a symbol.''The Oxford Companion to the English Language'' (1992) p. 709. Footnotes are informational notes located at the foot of the thematically relevant page, whilst endnotes are informational notes published at the end of a chapter, the end of a volume, or the conclusion of a multi-volume book. Unlike footnotes, which require manipulating the page design (text-block and page layouts) to accommodate the additional text, endnotes are advantageous to editorial production because the textual inclusion does not alter the design of the publication. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and experiments reported in an article must be successfully repeated in the laboratory of a member of the editorial board as a check for reproducibility prior to publication. The journal is published by Organic Syntheses, Inc., a non-profit corporation. An annual print version is published by John Wiley & Sons on behalf of Organic Syntheses, Inc. History Prior to World War I, work on synthetic organic chemistry in the United States had been quite limited, and most of the reagents used in laboratories had to be imported from Europe. When export stoppages and trade embargoes cut off this source, Clarence Derick, a professor of chemistry at University of Illinois at Urbana-Champaign, began an effort to synthesize these needed chemicals in industri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blast Shield
Blast or The Blast may refer to: *Explosion, a rapid increase in volume and release of energy in an extreme manner *Detonation, an exothermic front accelerating through a medium that eventually drives a shock front *A planned explosion in a mine, quarry or other situation in order to fragment rock Film * ''Blast'' (1997 film), starring Andrew Divoff * ''Blast'' (2000 film), starring Liesel Matthews * ''Blast'' (2004 film), an action comedy film * ''Blast!'' (1972 film) or ''The Final Comedown'', an American drama * ''BLAST!'' (2008 film), a documentary about the BLAST telescope * '' A Blast'', a 2014 film directed by Syllas Tzoumerkas Magazines * ''Blast'' (British magazine), a 1914–15 literary magazine of the Vorticist movement * ''Blast'' (U.S. magazine), a 1933–34 American short-story magazine * ''The Blast'' (magazine), a 1916–17 American anarchist periodical Music * Blast (American band), a hardcore punk band * Blast (Russian band), an indie band * Blxst, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition of a substance caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Since thermal decomposition is a kinetic process, the observed temperature of its beginning in most instances will be a function of the experimental conditions and sensitivity of the experimental setup. For a rigorous depiction of the process, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Phenylimide
Triphenylphosphine phenylimide is the organophosphorus compound with the formula Ph3P=NPh ( Ph = C6H5). The compound is classified as a phosphanimine. This white solid is soluble in organic solvents. The compound is a prototype of a large class of Staudinger reagents, resulting from the Staudinger reaction. The phosphanimines were first prepared in the laboratory of Nobelist Hermann Staudinger. His synthesis involved the direct reaction of triphenylphosphine with phenylazide. :Ph3P + N3Ph → Ph3P=NPh + N2 X-ray crystallography X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ... establishes that the P–N–C angle is bent (130.4°) and the P–N distance is 160 pm.{{cite journal, title=Die Kristallstrukturen von Ph3PNPh, h3PN(H)PhAuI2], und von 2,3-Bis(triphenylphospho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the thre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
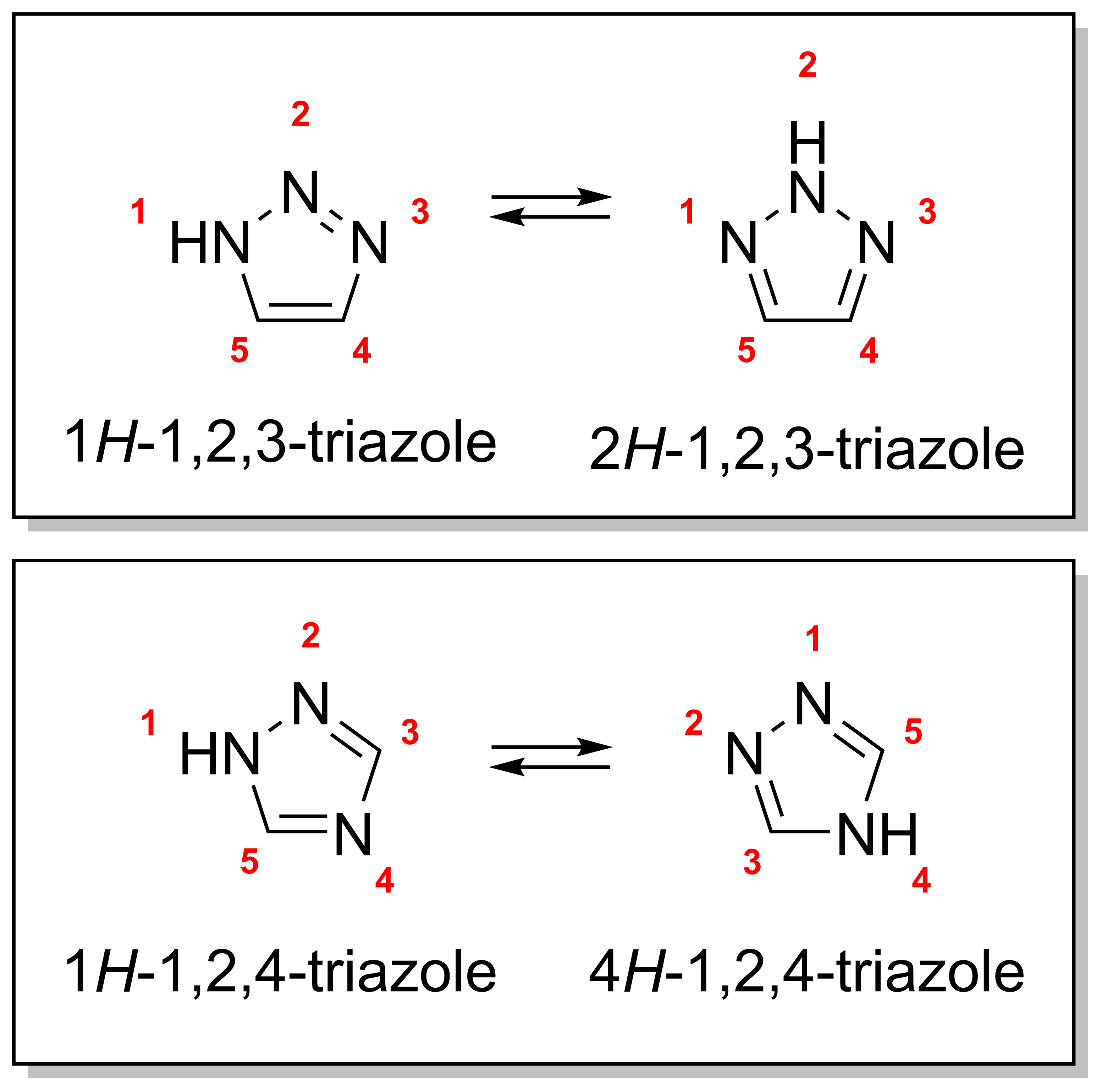
Triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial Isomer, isomerism, depending on the positioning of the nitrogen atoms within the ring. Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to Azide, azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as Piano stool complex, haptic ligands. Isomerism There are four triazole isomers, which are conventionally divided into two pairs of Tautomer, tautomers. In the 1,2,3-Triazole, 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-Triazole, 1,2,4-triazoles, an interstitial carbon separates out one nitrogen atom. Each category ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas. Preparation In the laboratory, phenylacetylene can be prepared by elimination of hydrogen bromide from styrene dibromide using sodium amide in ammonia: : It can also be prepared by the elimination of hydrogen bromide from bromostyrene using molten potassium hydroxide. Yet another method involves the Sonogashira coupling of iodobenzene with trimethylsilylacetylene, followed by removal of the trimethylsilyl group using TBAF. Reactions Phenylacetylene is a prototypical terminal acetylene, undergoing many reactions expected of that functional group. It undergoes semihydrogenation over Lindlar catalyst to give styrene. In the presence of base and copper(II) salts, it undergoes oxidative coupling to give diphenylbutadiyne. In the presence of metal cata ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Click Chemistry
Click chemistry is an approach to chemical synthesis that emphasizes efficiency, simplicity, selectivity, and modularity in chemical processes used to join molecular building blocks. It includes both the development and use of "click reactions", a set of simple, Biocompatibility, biocompatible chemical reactions that meet specific criteria like high yield, fast reaction rates, and minimal byproducts. It was first fully described by K. Barry Sharpless, Hartmuth C. Kolb, and M. G. Finn of Scripps Research Institute, The Scripps Research Institute in 2001. The paper argued that synthetic chemistry could emulate the way nature constructs complex molecules, using efficient reactions to join together simple, non-toxic building blocks. The term "click chemistry" was coined in 1998 by Sharpless' wife, Jan Dueser, who found the simplicity of this approach to chemical synthesis akin to clicking together Lego blocks. In fact, the simplicity of click chemistry represented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |