|
Click Chemistry
Click chemistry is an approach to chemical synthesis that emphasizes efficiency, simplicity, selectivity, and modularity in chemical processes used to join molecular building blocks. It includes both the development and use of "click reactions", a set of simple, Biocompatibility, biocompatible chemical reactions that meet specific criteria like high yield, fast reaction rates, and minimal byproducts. It was first fully described by K. Barry Sharpless, Hartmuth C. Kolb, and M. G. Finn of Scripps Research Institute, The Scripps Research Institute in 2001. The paper argued that synthetic chemistry could emulate the way nature constructs complex molecules, using efficient reactions to join together simple, non-toxic building blocks. The term "click chemistry" was coined in 1998 by Sharpless' wife, Jan Dueser, who found the simplicity of this approach to chemical synthesis akin to clicking together Lego blocks. In fact, the simplicity of click chemistry represented ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the human body and eventually how those interactions determine the clinical success of a medical device (such as pacemaker, hip replacement or stent). Modern medical devices and prostheses are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material. Since the immune response and repair functions in the body are so complicated it is not adequate to describe the biocompatibility of a single material in relation to a single cell type or tissue. Sometimes one hears of biocompatibility testing that is a large battery of in vitro test that is used in accordance with ISO 10993 (or other similar standards) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the Multiplicity (chemistry)#Molecules, bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are Concerted reaction, concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile. Cycloadditions can be described using two systems of notation. An older but still common notation is based on the size of linear arrangements of atoms in the reactants. It uses parentheses: where the variables are the numbers of linear atoms in each reactant. The product is a cycle of size . In this system, the standard Diels-Alder reaction is a (4 + 2)-cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Reaction
The aldol reaction (aldol addition) is a Chemical reaction, reaction in organic chemistry that combines two Carbonyl group, carbonyl compounds (e.g. aldehydes or ketones) to form a new β-hydroxy carbonyl compound. Its simplest form might involve the nucleophilic addition of an Enolate, enolized ketone to another: These products are known as ''aldols'', from the ''ald''ehyde + alcoh''ol'', a structural motif seen in many of the products. The use of aldehyde in the name comes from its history: aldehydes are more reactive than ketones, so that the reaction was discovered first with them. The aldol reaction is paradigmatic in organic chemistry and one of the most common means of forming carbon–carbon bonds in organic chemistry. It lends its name to the family of aldol reactions and similar techniques analyze a whole family of carbonyl α-substitution reactions, as well as the Claisen condensation, diketone condensations. Scope Aldol structural units are found in many importa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the cellular metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. ''Urea'' is Neo-Latin, , , itself from Proto-Indo-European ''*h₂worsom''. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor base (chemistry), alkaline. The body uses it in many processes, most notably metabolic waste#Nitrogen wastes, nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aziridines
220 px, chemotherapy.html" ;"title="Mitomycin C, an aziridine, is used as a chemotherapy">chemotherapeutic agent by virtue of its antitumour activity. In organic chemistry, aziridines are organic compounds containing the aziridine functional group (chemical structure ), a three-membered heterocycle with one amine () and two methylene bridges (). The parent compound is aziridine (or ethylene imine), with molecular formula . Several drugs feature aziridine rings, including zoldonrasib, thiotepa, mitomycin C, porfiromycin, and azinomycin B (carzinophilin). Structure The bond angles in aziridine are approximately 60°, considerably less than the normal hydrocarbon bond angle of 109.5°, which results in angle strain as in the comparable cyclopropane and ethylene oxide molecules. A banana bond model explains bonding in such compounds. Aziridine is less basic than acyclic aliphatic amines, with a pKa of 7.9 for the conjugate acid, due to increased s character of the nitrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxy
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called ''epoxy''. The IUPAC name for an epoxide group is an oxirane. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols, alcohols and thiols (sometimes called mercaptans). These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as Curing (chemistry), curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable mechanical properties and high thermal and chemical resistance. Epoxy has a wide range of application ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some Stress (mechanics), stress which raises its internal energy in comparison to a strain-free reference Chemical compound, compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compression (physics), compressed spring (device), spring.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the Chemical bond, bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released. Summary Thermodynamics The Chemical equilibrium, equilibrium of two c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
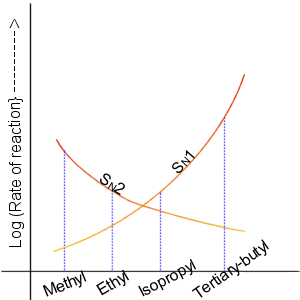
Nucleophilic Substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic & Biomolecular Chemistry
Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product of decay, or is composed of organic compounds * Organic compound, a compound that contains carbon ** Organic chemistry, chemistry involving organic compounds Farming, certification and products * Organic farming, agriculture conducted according to certain standards, especially the use of stated methods of fertilization and pest control * Organic certification, accreditation process for producers of organically-farmed products * Organic horticulture, the science and art of growing fruits, vegetables, flowers, or ornamental plants by following the essential principles of organic agriculture * Organic products, "organics": ** Organic food, food produced from organic farming methods and often certified organic according to organic farming stand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioconjugate Chemistry (journal)
''Bioconjugate Chemistry'' is a monthly peer-reviewed scientific journal covering research in bioconjugation and the interface between man-made and biological materials. The journal was established in 1990 and is published by the American Chemical Society. It is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/MEDLINE/PubMed, and the Science Citation Index Expanded. The current editor-in-chief is Theresa M. Reineke (University of Minnesota). pubs.acs.org. Retrieved on 2023-11-22. According to the '''', the journal has a 2022 |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira (ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2023 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a type of journal ranking. Journals with higher impact factor values are considered more prestigious or important within their field. The Impact Factor of a journa ... of 14.4. Editors-in-chief The following people are or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inverse Electron Demand Diels-Alder Reaction
Inverse or invert may refer to: Science and mathematics * Inverse (logic), a type of conditional sentence which is an immediate inference made from another conditional sentence * Additive inverse, the inverse of a number that, when added to the original number, yields zero * Compositional inverse, a function that "reverses" another function * Inverse element * Inverse function, a function that "reverses" another function **Generalized inverse, a matrix that has some properties of the inverse matrix but not necessarily all of them * Multiplicative inverse (reciprocal), a number which when multiplied by a given number yields the multiplicative identity, 1 ** Inverse matrix of an Invertible matrix Other uses * Invert level, the base interior level of a pipe, trench or tunnel * ''Inverse'' (website), an online magazine * An outdated term for an LGBT person; see Sexual inversion (sexology) See also * Inversion (other) * Inverter (other) * Opposite (disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |