|
PRELP
Prolargin is a protein that in humans is encoded by the ''PRELP'' gene. The protein encoded by this gene is a leucine-rich repeat protein present in connective tissue extracellular matrix. This protein functions as a molecule anchoring basement membranes to the underlying connective tissue. This protein has been shown to bind type I collagen to basement membranes and type II collagen to cartilage. It also binds the basement membrane heparan sulfate proteoglycan perlecan. This protein is suggested to be involved in the pathogenesis of Hutchinson–Gilford progeria (HGP), which is reported to lack the binding of collagen in basement membranes and cartilage. Alternatively spliced Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be i ... transcript variants encoding the same protein have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine-rich Repeat
A leucine-rich repeat (LRR) is a protein structural motif that forms an α/β horseshoe fold. It is composed of repeating 20–30 amino acid stretches that are unusually rich in the hydrophobic amino acid leucine. These tandem repeats commonly fold together to form a solenoid protein domain, termed leucine-rich repeat domain. Typically, each repeat unit has beta strand-turn-alpha helix structure, and the assembled domain, composed of many such repeats, has a horseshoe shape with an interior parallel beta sheet and an exterior array of helices. One face of the beta sheet and one side of the helix array are exposed to solvent and are therefore dominated by hydrophilic residues. The region between the helices and sheets is the protein's hydrophobic core and is tightly sterically packed with leucine residues. Leucine-rich repeats are frequently involved in the formation of protein–protein interactions. Examples Leucine-rich repeat motifs have been identified in a large num ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Connective Tissue
Connective tissue is one of the four primary types of animal tissue, along with epithelial tissue, muscle tissue, and nervous tissue. It develops from the mesenchyme derived from the mesoderm the middle embryonic germ layer. Connective tissue is found in between other tissues everywhere in the body, including the nervous system. The three meninges, membranes that envelop the brain and spinal cord are composed of connective tissue. Most types of connective tissue consists of three main components: elastic and collagen fibers, ground substance, and cells. Blood, and lymph are classed as specialized fluid connective tissues that do not contain fiber. All are immersed in the body water. The cells of connective tissue include fibroblasts, adipocytes, macrophages, mast cells and leucocytes. The term "connective tissue" (in German, ''Bindegewebe'') was introduced in 1830 by Johannes Peter Müller. The tissue was already recognized as a distinct class in the 18th century. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. Each type of conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basement Membrane
The basement membrane is a thin, pliable sheet-like type of extracellular matrix that provides cell and tissue support and acts as a platform for complex signalling. The basement membrane sits between epithelial tissues including mesothelium and endothelium, and the underlying connective tissue. Structure As seen with the electron microscope, the basement membrane is composed of two layers, the basal lamina and the reticular lamina. The underlying connective tissue attaches to the basal lamina with collagen VII anchoring fibrils and fibrillin microfibrils. The basal lamina layer can further be subdivided into two layers based on their visual appearance in electron microscopy. The lighter-colored layer closer to the epithelium is called the lamina lucida, while the denser-colored layer closer to the connective tissue is called the lamina densa. The electron-dense lamina densa layer is about 30–70 nanometers thick and consists of an underlying network of reticular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type I Collagen
Type I collagen is the most abundant collagen of the human body. It forms large, eosinophilic fibers known as collagen fibers. It is present in scar tissue, the end product when tissue heals by repair, as well as tendons, ligaments, the endomysium of myofibrils, the organic part of bone, the dermis, the dentin, and organ capsules. Formation The gene produces the pro-alpha1(I) chain. This chain combines with another pro-alpha1(I) chain and also with a pro-alpha2(I) chain (produced by the gene) to make a molecule of type I pro-collagen. These triple-stranded, rope-like pro-collagen molecules must be processed by enzymes outside the cell. Once these molecules are processed, they arrange themselves into long, thin fibrils that cross-link to one another in the spaces around cells. The cross-links result in the formation of very strong mature type I collagen fiber. Clinical significance See Collagen, type I, alpha 1#Clinical significance Markers used to measure bone loss are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type II Collagen
Type II collagen is the basis for hyaline cartilage, including the articular cartilages at joint surfaces. It is formed by homotrimers of collagen, type II, alpha 1 chains. It makes up 50% of all protein in cartilage and 85–90% of collagen of articular cartilage. Type II collagen is organised into fibrils. This fibrillar network of collagen allows the cartilage to entrap the proteoglycan aggregate, as well as providing tensile strength to the tissue. Oral administration of native type II collagen induces oral tolerance to pathological immune responses and may be useful in arthritis. See also * Type I collagen * Collagen, type III, alpha 1 Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded ... References External links * Collagens {{gene-12-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
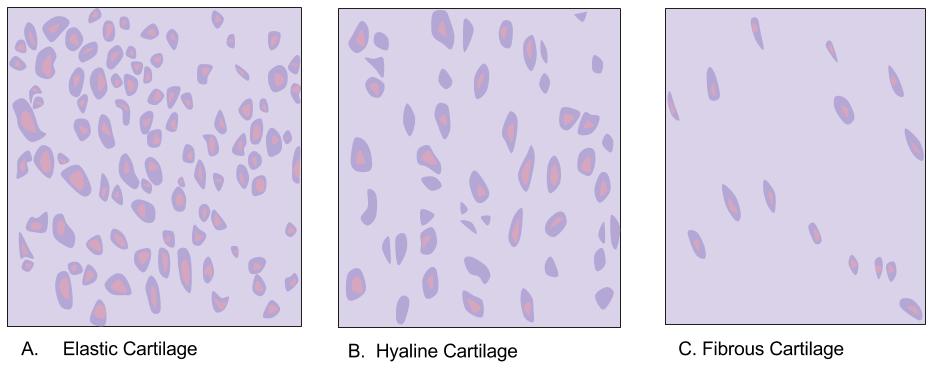
Cartilage
Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin. Because of its rigidity, cartilage often serves the purpose of holding tubes open in the body. Examples include the rings of the trachea, such as the cricoid cartilage and carina. Cartilage is composed of specialized cells called chondrocytes that produce a large amount of collagenous extracellular matrix, abundant ground substance that is rich in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heparan Sulfate
Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan (HSPG, i.e. Heparan Sulfate ProteoGlycan) in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. It is in this form that HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB (Granzyme B), and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2. Proteoglycans The major cell membrane HSPGs are the transmembrane syndecans and the glycosylphosphatidylinositol (GPI) anchored glypicans. Other minor forms of membrane HSPG include betaglycan and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate-GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser- Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perlecan
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the ''HSPG2'' gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins. Perlecan was originally isolated from a tumor cell line and shown to be present in all native basement membranes. Perlecan is a large multidomain (five domains, labeled I-V) proteoglycan that binds to and cross-links many extracellular matrix (ECM) components and cell-surface molecules. Perlecan is synthesized by both vascular endothelial and smooth muscle cells and deposited in the extracellular matrix of parahoxozoans. Perlecan is highly conserved across species and the available data indicate that it has evolved from ancient ancestors by gene duplication and exon shuffling. Structure Perlecan consists of a core protein of molecular weight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |