|
Type II Collagen
Type II collagen is the basis for hyaline cartilage, including the articular cartilages at joint surfaces. It is formed by homotrimers of collagen, type II, alpha 1 chains. It makes up 50% of all protein in cartilage and 85–90% of collagen of articular cartilage. Type II collagen is organised into fibrils. This fibrillar network of collagen allows the cartilage to entrap the proteoglycan aggregate, as well as providing tensile strength to the tissue. Oral administration of native type II collagen induces oral tolerance to pathological immune responses and the administration of type II collagen tablets together with paracetamol might be more effective at reducing symptoms of osteoarthritis than paracetamol by itself. See also * Type I collagen Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type II, Alpha 1
Collagen, type II, alpha 1 (primary osteoarthritis, spondyloepiphyseal dysplasia, congenital), also known as COL2A1, is a human gene that provides instructions for the production of the pro-alpha1(II) chain of type II collagen. Gene The COL2A1 gene is located on the long (q) arm of chromosome 12 between positions 13.11 and 13.2, from base pair 46,653,017 to base pair 46,684,527. The expression of COL2A1 is regulated by SOX-9 and retrotransposon gag-like-3 gene RTL3 in chondrocytes. There are two transcripts identified for this gene. Function This gene encodes the alpha-1 chain of type II collagen, a fibrillar collagen found in cartilage and the vitreous humor of the eye. Type II collagen, which adds structure and strength to connective tissues, is found primarily in cartilage, the jelly-like substance that fills the eyeball (the vitreous), the inner ear, and the center portion of the discs between the vertebrae in the spine (nucleus pulposus). Three pro-alpha1(II) chains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
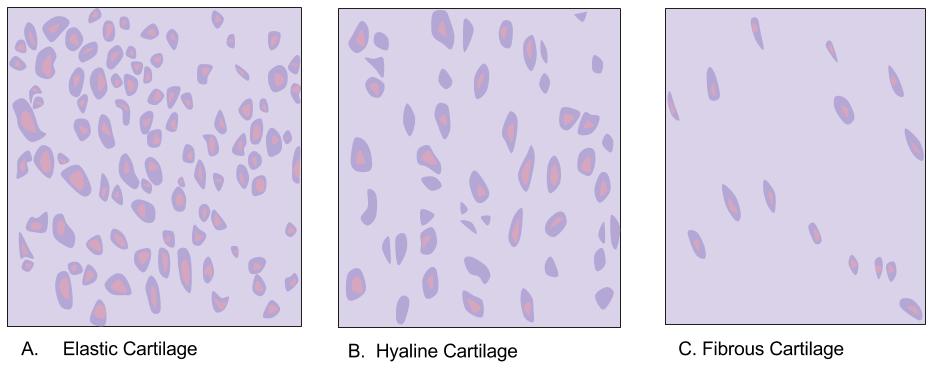
Hyaline Cartilage
Hyaline cartilage is the glass-like (hyaline) and translucent cartilage found on many joint surfaces. It is also most commonly found in the ribs, nose, larynx, and trachea. Hyaline cartilage is pearl-gray in color, with a firm consistency and has a considerable amount of collagen. It contains no nerves or blood vessels, and its structure is relatively simple. Structure Hyaline cartilage is the most common kind of cartilage in the human body. It is primarily composed of type II collagen and proteoglycans. Hyaline cartilage is located in the trachea, nose, epiphyseal plate, sternum, and ribs. Hyaline cartilage is covered externally by a fibrous membrane known as the perichondrium. The primary cells of cartilage are chondrocytes, which are in a matrix of fibrous tissue, proteoglycans and glycosaminoglycans. As cartilage does not have lymph glands or blood vessels, the movements of solutes, including nutrients, occur via diffusion within the fluid compartments contiguous with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type II, Alpha 1
Collagen, type II, alpha 1 (primary osteoarthritis, spondyloepiphyseal dysplasia, congenital), also known as COL2A1, is a human gene that provides instructions for the production of the pro-alpha1(II) chain of type II collagen. Gene The COL2A1 gene is located on the long (q) arm of chromosome 12 between positions 13.11 and 13.2, from base pair 46,653,017 to base pair 46,684,527. The expression of COL2A1 is regulated by SOX-9 and retrotransposon gag-like-3 gene RTL3 in chondrocytes. There are two transcripts identified for this gene. Function This gene encodes the alpha-1 chain of type II collagen, a fibrillar collagen found in cartilage and the vitreous humor of the eye. Type II collagen, which adds structure and strength to connective tissues, is found primarily in cartilage, the jelly-like substance that fills the eyeball (the vitreous), the inner ear, and the center portion of the discs between the vertebrae in the spine (nucleus pulposus). Three pro-alpha1(II) chains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cartilage
Cartilage is a resilient and smooth type of connective tissue. Semi-transparent and non-porous, it is usually covered by a tough and fibrous membrane called perichondrium. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans and cyclostomes, it constitutes a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin. It usually grows quicker than bone. Because of its rigidity, cartilage often serves the purpose of holding tubes open in the body. Examples include the rings of the trachea, such as the cricoid cartilage and carina. Cartilage is composed of specialized c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibril
Fibrils () are structural biological materials found in nearly all living organisms. Not to be confused with fibers or protein filament, filaments, fibrils tend to have diameters ranging from 10 to 100 nanometers (whereas fibers are micro to milli-scale structures and filaments have diameters approximately 10–50 nanometers in size). Fibrils are not usually found alone but rather are parts of greater hierarchical structures commonly found in biological systems. Due to the prevalence of fibrils in biological systems, their study is of great importance in the fields of microbiology, biomechanics, and materials science. Structure and mechanics Fibrils are composed of linear biopolymers, and are characterized by rod-like structures with high length-to-diameter ratios. They often spontaneously arrange into helical structures. In biomechanics problems, fibrils can be characterized as classical beams with a roughly circular cross-sectional area on the nanometer scale. As such, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis. Depending on the degree of biomineralization, mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the Gut (anatomy), gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1% to 2% of muscle tissue and 6% by weight of skeletal muscle. The fibroblast is the most common cell creating collagen in animals. Gelatin, which is used in food and industry, is collagen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser- Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type I Collagen
Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I forms large, eosinophilic fibers known as collagen fibers, which make up most of the rope-like dense connective tissue in the body. Collagen I itself is created by the combination of both a proalpha1 and a proalpha2 chain created by the COL1alpha1 and COL1alpha2 genes respectively. The Col I gene itself takes up a triple-helical conformation due to its Glycine-X-Y structure, x and y being any type of amino acid. Collagen can also be found in two different isoforms, either as a homotrimer or a heterotrimer, both of which can be found during different periods of development. Heterotrimers, in particular, play an important role in wound healing, and are the dominant isoform found in the body. Type I collagen can be found in a myriad of different places in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type III, Alpha 1
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain. Gene The gene is located on the long (q) arm of chromosome 2 at 2q32.2, between positions and . The gene has 51 exons and is approximately 40 kbp long. The ''COL3A1'' gene is in tail-to-tail orientation with a gene for another fibrillar collagen, namely . Two transcripts are generated from the gene using different polyadenylation sites. Although alternatively spliced transcripts have been detected for this gene, they are the result of mutations; these mutations alter RNA splicing, often leading to the exclusion of an exon or use of cryptic splice sites. The resulting defective protein is the cause o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |