|
PECVD
Plasma-enhanced chemical vapor deposition (PECVD) is a chemical vapor deposition process used to deposit thin films from a gas state (vapor) to a solid state on a substrate (materials science), substrate. Chemical reactions are involved in the process, which occur after creation of a plasma (physics), plasma of the reacting gases. The plasma (physics), plasma is generally created by radio frequency (RF) alternating current (AC) frequency or direct current (DC) discharge between two electrodes, the space between which is filled with the reacting gases. Discharges for processes A plasma is any gas in which a significant percentage of the atoms or molecules are ionized. Fractional ionization in plasmas used for deposition and related materials processing varies from about 10−4 in typical capacitive discharges to as high as 5–10% in high-density inductive plasmas. Processing plasmas are typically operated at pressures of a few millitorrs to a few torr, although arc discharges an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LEPECVD
Low-energy plasma-enhanced chemical vapor deposition (LEPECVD) is a plasma-enhanced chemical vapor deposition technique used for the epitaxy, epitaxial deposition of thin semiconductor (silicon, germanium and Silicon-germanium, SiGe alloys) films. A remote low energy, high density direct current, DC argon Plasma (physics), plasma is employed to efficiently decompose the gas phase Precursor (chemistry), precursors while leaving the epitaxial layer undamaged, resulting in high quality epilayers and high deposition rates (up to 10 nm/s). Working principle The Substrate (materials science), substrate (typically a silicon Wafer (electronics), wafer) is inserted in the reactor chamber, where it is heated by a graphite resistive heater from the backside. An argon plasma is introduced into the chamber to ionize the precursors' molecules, generating highly reactive Radical (chemistry), radicals which result in the growth of an epilayer on the substrate. Moreover, the bombardment of Ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films. In typical CVD, the wafer (electronics), wafer (substrate) is exposed to one or more Volatility (chemistry), volatile wikt:precursor, precursors, which chemical reaction, react and/or chemical decomposition, decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. Microfabrication processes widely use CVD to deposit materials in various forms, including: Single crystal, monocrystalline, polycrystalline, amorphous, and Epitaxy, epitaxial. These materials include: silicon (Silicon dioxide, dioxide, silicon carbide, carbide, silicon nitride, nitride, silicon oxynitride, oxynitride), carbon (carbon (fiber), fiber, carbon nanofibers, nanofibers, carbon nanot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
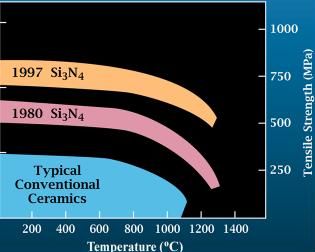
Silicon Nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. (''Trisilicon tetranitride'') is the most thermodynamically stable and commercially important of the silicon nitrides, and the term ″''Silicon nitride''″ commonly refers to this specific composition. It is a white, high-melting-point solid that is relatively chemically inert, being attacked by dilute HF and hot . It is very hard (8.5 on the mohs scale). It has a high thermal stability with strong optical nonlinearities for all-optical applications. Production Silicon nitride is prepared by heating powdered silicon between 1300 °C and 1400 °C in a nitrogen atmosphere: :3 Si + 2 → The silicon sample weight increases progressively due to the chemical combination of silicon and nitrogen. Without an iron catalyst, the reaction is complete after several hours (~7), when no further weight increase due to nitrogen absorption (per gram of silicon) is detected. In addition to , several other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silane
Silane (Silicane) is an inorganic compound with chemical formula . It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silanes with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : The trichlorosilane is then converted to a mixture ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
STS Multiplex PECVD Machine At LAAS 0495
STS, or sts, may refer to: Medicine * Secondary traumatic stress, a condition which leads to a diminished ability to empathize * Sequence-tagged site, a gene-reference in genomics * Soft-tissue sarcoma * Staurosporine, an antibiotic * STS (gene), which codes for steroid sulfatase * Superior temporal sulcus * Sinus tarsi syndrome, a foot condition Places * Semipalatinsk Test Site for Soviet nuclear weapons * Staffordshire, county in England, Chapman code Transport * Cadillac STS, a luxury car * NASA Space Transportation System, the system in which the NASA shuttle is part of and only surviving component of; starting as a 1969 NASA proposal system for reusable space vehicles ** NASA Space Shuttle program, the shuttle program itself, whose mission were referred to with STS-numbering * Sail training ship, a ship prefix * Satellite Transit System, now called the SEA Underground, airport transit in Seattle-Tacoma International Airport * Ship-to-ship transfer, between seagoing ships * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an List of extremely hazardous substances, extremely hazardous substance. Ammonia is toxic, cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrous Oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or factitious air, among others, is a chemical compound, an Nitrogen oxide, oxide of nitrogen with the Chemical formula, formula . At room temperature, it is a colourless Flammability#Definitions, non-flammable gas, and has a slightly sweet scent and taste. At elevated temperatures, nitrous oxide is a powerful Oxidising agent, oxidiser similar to molecular oxygen. Nitrous oxide has significant Nitrous oxide (medication), medical uses, especially in surgery and dentistry, for its Anesthesia, anaesthetic and Analgesic, pain-reducing effects, and it is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Its colloquial name, "laughing gas", coined by Humphry Davy, describes the Euphoria, euphoric effects upon inhaling it, which cause it to be used as a recreational drug inducing a brief "Dissociative, high". When abused chronically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), nonmetal, and a potent oxidizing agent that readily forms oxides with most elements as well as with other chemical compound, compounds. Oxygen is abundance of elements in Earth's crust, the most abundant element in Earth's crust, making up almost half of the Earth's crust in the form of various oxides such as water, carbon dioxide, iron oxides and silicates.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. It is abundance of chemical elements, the third-most abundant element in the universe after hydrogen and helium. At standard temperature and pressure, two oxygen atoms will chemical bond, bind covalent bond, covalently to form dioxygen, a colorless and odorless diatomic gas with the chemical formula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorosilane
Dichlorosilane, or DCS as it is commonly known, is a chemical compound with the formula H2SiCl2. In its major use, it is mixed with ammonia (NH3) in LPCVD chambers to grow silicon nitride in semiconductor processing. A higher concentration of DCS·NH3 (i.e. 16:1), usually results in lower stress nitride films. History Dichlorosilane was originally prepared by Stock and Somieski by the reaction of SiH4 with hydrogen chloride. Dichlorosilane reacts with water vapor to initially give monomeric prosiloxane: Monomeric polymerizes rapidly upon condensation or in solution.Seyferth, D., Prud'Homme, C., Wiseman, G., Cyclic Polysiloxanes from the Hydrolysis of Dichlorosilane, Inorganic Chemistry, 22, 2163-2167 Reactions and formation Most dichlorosilane results as a byproduct of the reaction of HCl with silicon, a reaction intended to give trichlorosilane. Disproportionation In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |