|
Lithium Selenide
Lithium selenide is an inorganic compound that formed by selenium and lithium. It is a selenide with a chemical formula Li2Se. Lithium selenide has the same crystal form as other selenides, which is cubic, belonging to the anti-fluorite structure, the space group is Fm\barm, each unit cell has 4 units. References Lithium compounds Selenides Fluorite crystal structure {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Polonide
Lithium polonide is a chemical compound with the formula Li2 Po. It is a polonide, a set of very chemically stable compounds of polonium.. Production Lithium polonide may be produced from a redox reaction between aqueous hydrogen polonide and lithium metal or from an acid-base reaction of H2Po with strong lithium-containing bases: :H2Po + 2 Li → Li2Po + H2 It may also be produced by heating lithium and polonium together at 300–400 °C. Crystal structure Like sodium polonide Sodium polonide is a radioactive chemical compound with the formula Na2 Po. This salt is a polonide, a set of very chemically stable compounds of polonium.. Due to the difference in electronegativity (ΔEN) between sodium and polonium (≈ 1.1 ..., lithium polonide has the antifluorite structure. References {{inorganic-chemistry-stub Lithium compounds Polonides Fluorite crystal structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Compounds
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space Group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchanged. In three dimensions, space groups are classified into 219 distinct types, or 230 types if chiral copies are considered distinct. Space groups are discrete cocompact groups of isometries of an oriented Euclidean space in any number of dimensions. In dimensions other than 3, they are sometimes called Bieberbach groups. In crystallography, space groups are also called the crystallographic or Fedorov groups, and represent a description of the symmetry of the crystal. A definitive source regarding 3-dimensional space groups is the ''International Tables for Crystallography'' . History Space groups in 2 dimensions are the 17 wallpaper groups which have been known for several centuries, though the proof that the list was compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
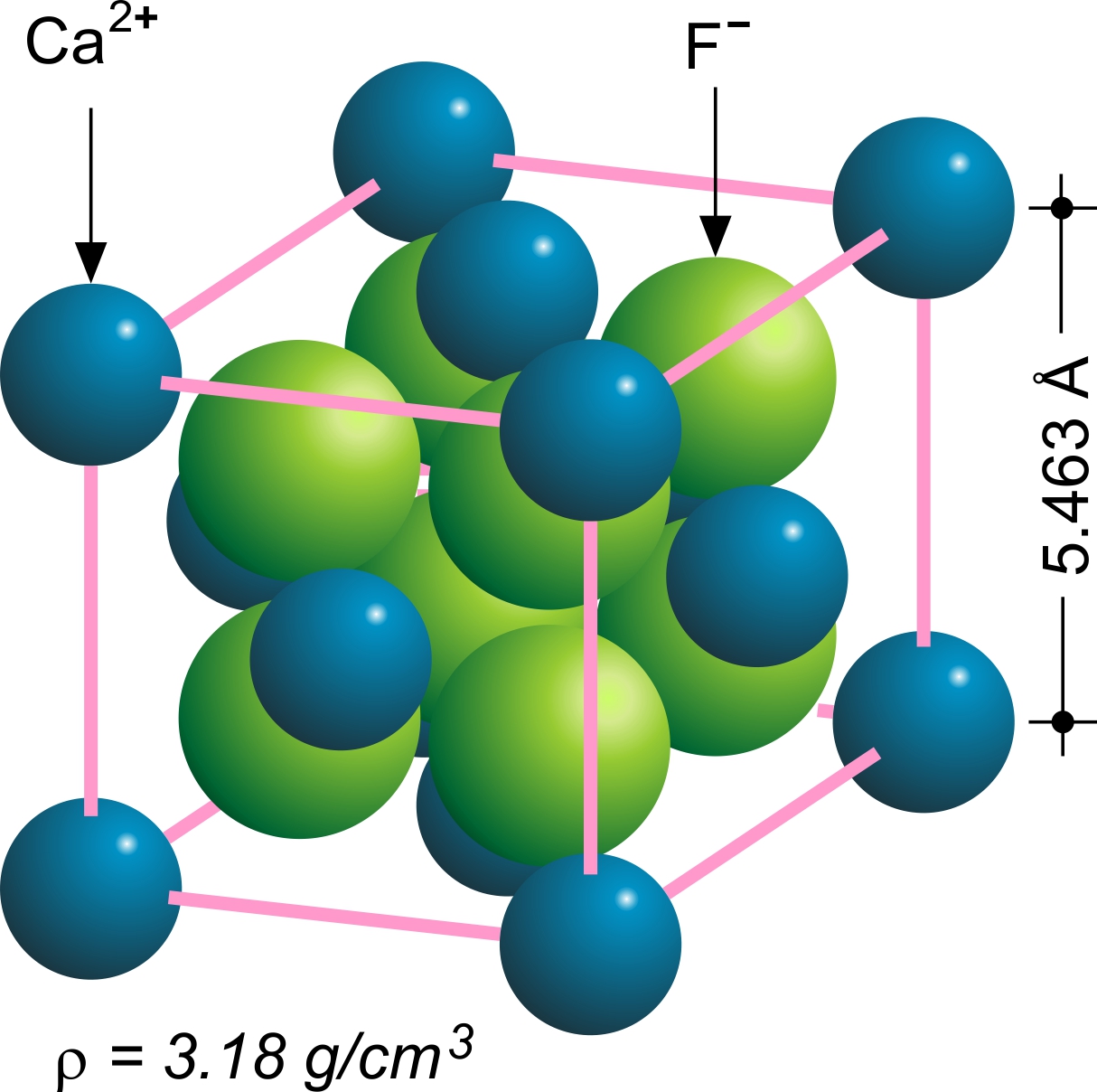
Fluorite Structure
In solid state chemistry, the fluorite structure refers to a common motif for compounds with the formula MX2. The X ions occupy the eight tetrahedral interstitial sites whereas M ions occupy the regular sites of a face-centered cubic (FCC) structure. Many compounds, notably the common mineral fluorite (CaF2), adopt this structure. Many compounds with formula M2X have an antifluorite structure. In these the locations of the anions and cations are reversed relative to fluorite (an anti-structure); the anions occupy the FCC regular sites whereas the cations occupy the tetrahedral interstitial sites. For example, Magnesium silicide, Mg2Si, has a lattice parameter of 6.338 Å with magnesium cations occupying the tetrahedral interstitial sites, in which each silicide anion is surrounded by eight magnesium cations and each magnesium cation is surrounded by four silicide anions in a tetrahedral fashion. File:Fluorite Structure.jpg, The fluorite structure of calcium fluoride CaF2. Fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenide
A selenide is a chemical compound containing a selenium anion with oxidation number of −2 (Se2−), much as sulfur does in a sulfide. The chemistry of the selenides and sulfides is similar. Similar to sulfide, in aqueous solution, the selenide ion, Se2−, is prevalent only in very basic conditions. In neutral conditions, hydrogen selenide ion, HSe−, is most common. In acid conditions, hydrogen selenide, H2Se, is formed. Some selenides are reactive to oxidation by air. Owing to the greater reducing power of selenide, metal selenides are more easily decomposed to the elements than are sulfides ( tellurides are even more labile). Selenides of electropositive metals: such as aluminium selenide readily hydrolyse, even in moist air, evolving toxic hydrogen selenide gas. Pure selenide minerals are rare, instead selenium tends to partially substitute for sulfide in many sulfide minerals. The degree of substitution is only of commercial interest for copper sulfide ores, in which c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium – from Greek ( 'Moon') – was discovered in 1817 by , who noted the similarity of the new element to the previously discovered tellurium (named for the Earth). Selenium is found in metal sulfide ores, where it partially replaces the sulfur. Commercially, selenium is produced as a byproduct in the refining of these ores, most often during production. Minerals that are pure selenide or selenate compounds are known but rare. The chief commercial uses for selenium today are glassmaking and pigments. Selenium is a semiconductor and is used in photocells. Applications in electronics, once important, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Telluride
Lithium telluride (Li2Te) is an inorganic compound of lithium and tellurium. Along with LiTe3, it is one of the two intermediate solid phases in the lithium-tellurium system. It can be prepared by directly reacting lithium and tellurium in a beryllium oxide Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is a notable electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and exceeds that of ... crucible at 950°C. References Lithium compounds Tellurides Fluorite crystal structure {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Sulfide
Lithium sulfide is the inorganic compound with the formula Li2 S. It crystallizes in the antifluorite motif, described as the salt (Li+)2S2−. It forms a solid yellow-white deliquescent powder. In air, it easily hydrolyses to release hydrogen sulfide (rotten egg odor). Preparation Lithium sulfide is prepared by treating lithium with sulfur. This reaction is conveniently conducted in anhydrous ammonia. :2 Li + S → Li2S The THF-soluble triethylborane adduct of lithium sulfide can be generated using superhydride Lithium triethylborohydride is the organoboron compound with the formula Li Et3 BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid b .... Reactions and applications Lithium sulfide has been considered for use in lithium–sulfur batteries. References External links Lithium Sulfide {{Sulfides Lithium salts Sulfides Fluorite crystal structure< ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Oxide
Lithium oxide ( O) or lithia is an inorganic chemical compound. It is a white solid. Although not specifically important, many materials are assessed on the basis of their Li2O content. For example, the Li2O content of the principal lithium mineral spodumene (LiAlSi2O6) is 8.03%. Production Lithium oxide is produced by thermal decomposition of lithium peroxide at 300–400 °C.Wietelmann, Ulrich and Bauer, Richard J. (2005) "Lithium and Lithium Compounds" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH: Weinheim. . Lithium oxide forms along with small amounts of lithium peroxide when lithium metal is burned in the air at and combines with oxygen at temperatures above 100 °C: :4Li + → 2. Pure can be produced by the thermal decomposition of lithium peroxide, , at 450 °C :2 → 2 + Structure Solid lithium oxide adopts an antifluorite structure with four-coordinated Li+ centers and eight-coordinated oxides. The ground state gas phase molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |